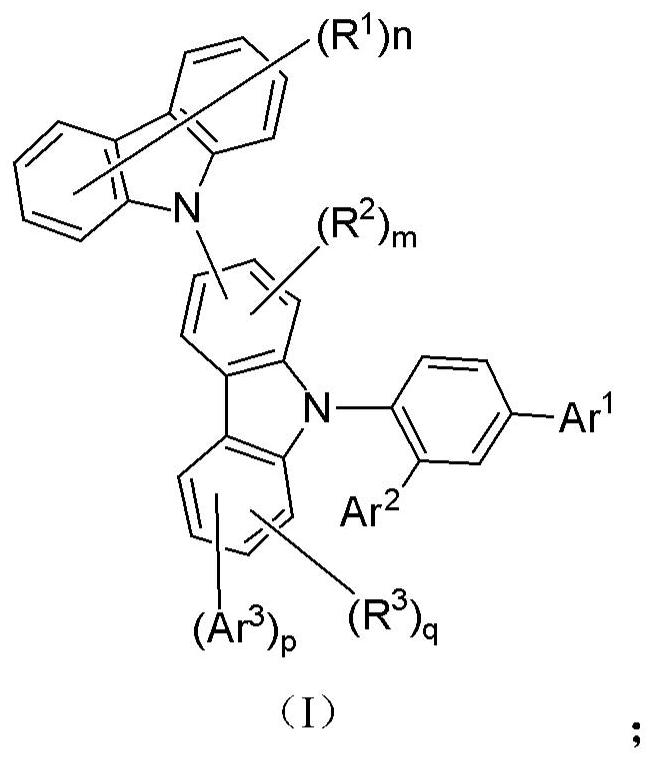
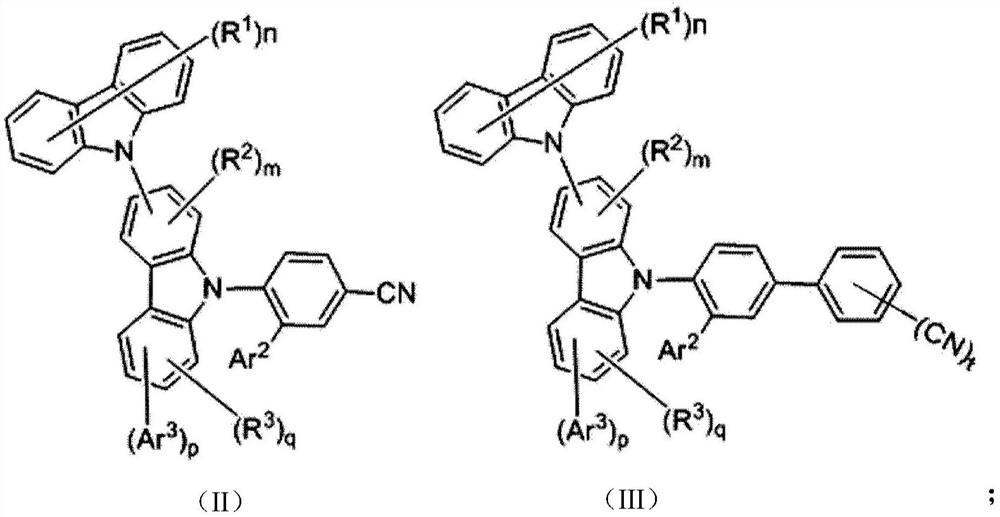
Compound, thermal activation delayed fluorescence material, organic electroluminescent device and application thereof
A compound and electromechanical technology, applied in the direction of luminescent materials, electrical solid devices, silicon organic compounds, etc., can solve the problems of improvement, instability of phosphorus-oxygen groups, inability to have both device efficiency and lifespan, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0065] Synthesis Example 1: Synthesis of Product P1
[0066]
[0067] Synthesis of Intermediate M1
[0068] Under nitrogen atmosphere, 3-bromo-4-fluorobenzonitrile 4.0g (19.8mmol, 1.1eq), 3,9-bicarbazole 6.0g (18mmol, 1.0eq), cesium carbonate 12.0g (36mmol, 2.0 eq), after adding 60 mL of DMF, the temperature was raised to 150° C., and the reaction was carried out overnight. Using silica gel column chromatography. Obtained M1, mass spectrum: 512.
[0069] Synthesis of Product P1
[0070] Under nitrogen atmosphere, M15.2g (10.0mmol, 1.0eq), 2,4-diphenyl-6-pinacol ester-1,3,5-triazine 4.4g (12.0mmol, 1.2eq), Pd(PPh 3 ) 4 0.23g (0.2mmol, 2%eq), potassium carbonate 4.2g (30.0mmol, 3eq) was added to the 100mL single-necked bottle, dioxane / water (50mL / 5mL) was added to the 100mL single-necked bottle, and the temperature was raised to reflux temperature, and the reaction overnight. Cool down to room temperature and filter the solid. After the solid is rinsed with a mixt...
Synthetic example 2
[0071] Synthesis Example 2: Synthesis of Product P9
[0072]
[0073] Synthesis of intermediate M2
[0074] The reactant 3-bromo-4-fluorobenzonitrile is replaced by 6-formonitrile-3',6'-dimethyl-9H-3,9-bicarbazole, and the process is the same as that of intermediate M1 in Synthesis Example 1 Synthetic method to get M2, mass spectrum: 565.
[0075] Synthesis of Product P9
[0076] The reactant M1 was replaced by the intermediate M2, and P9 was obtained through the same synthesis method as that of P1 in Synthesis Example 1, mass spectrum: 717. 1 H NMR (300MHz, CDCl 3 ): 8.72(1H), 8.30(4H), 8.15(1H), 7.99(1H), 7.86(1H), 7.81(2H), 7.62(2H), 7.51(5H), 7.40(3H), 7.32(2H ), 6.88(1H), 2.34(6H).
Synthetic example 3
[0077] Synthesis Example 3: Synthesis of Product P13
[0078]
[0079] Synthesis of Intermediate M3
[0080] Replacing reactant M1 with 2,4-diphenyl-6-pinacol ester-1,3,5-triazine 4-cyanophenylboronic acid with 6-bromo-3',6'-dimethyl -9H-3,9-bicarbazole, through the same synthesis method as product P1 in Synthesis Example 1, M3 was obtained, mass spectrum: 461.
[0081] Synthesis of Intermediate M4
[0082] The reactant 3,9-bicarbazole was replaced by intermediate M4, and M4 was obtained through the same synthesis method as intermediate M1 in Synthesis Example 1, mass spectrum: 641.
[0083] Synthesis of Product P13
[0084] The reactant M1 was replaced by the intermediate M4 for reaction, and P13 was obtained through the same synthesis method as the product P1 in Synthesis Example 1, mass spectrum: 793. 1 H NMR (300MHz, CDCl 3 ): 8.72(1H), 8.30(4H), 8.18(1H), 7.99(3H), 7.92(1H), 7.86(1H), 7.84(2H), 7.82(3H), 7.78(1H), 7.63(1H ), 7.61(1H), 7.51(5H), 7.40(3H), 7....
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com