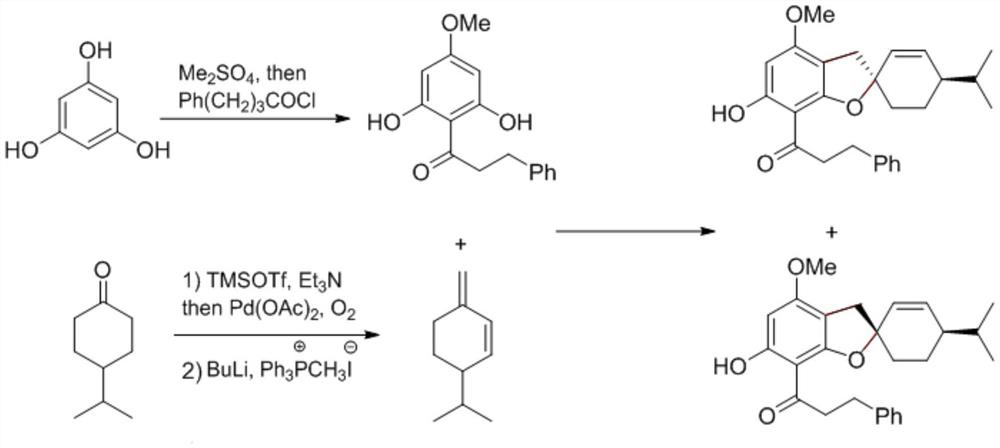
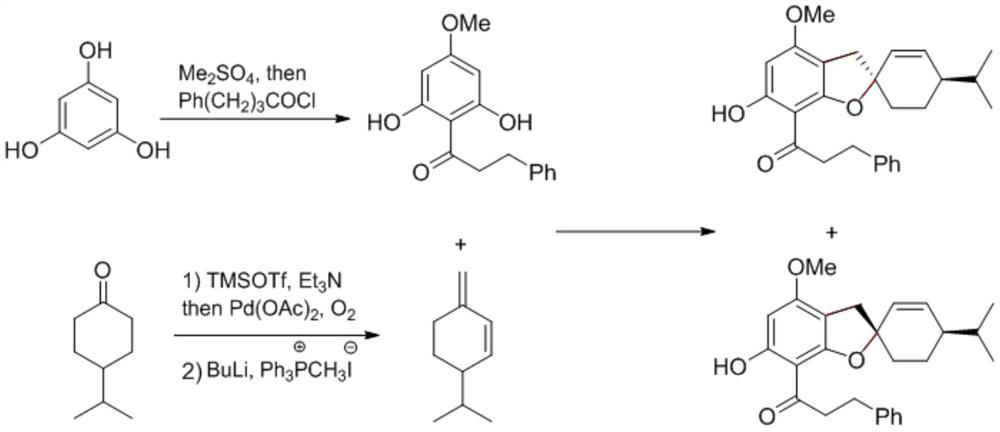
Preparation method of spirobenzofuran compounds
A compound, furan technology, applied in the field of chemical biomimetic synthesis of natural medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0027] A 300mL round bottom flask was preheated with an alcohol lamp to remove water for 10 minutes, during which the argon gas was replaced three times. Phlorotriphenol (99%, 12.7 g, 100 mmol) and anhydrous potassium carbonate (99%, 13.8 g, 100 mmol, 1.0 equiv) were added rapidly; the argon was replaced three times again. Add anhydrous acetone (100 mL, commercially purchased acetone needs to be pre-dried with anhydrous calcium chloride, and then distilled at atmospheric pressure for later use). Finally, dimethyl sulfate (99%, 3.2ml, 33mmol, 0.33equiv; protective measures must be taken during the process of adding dimethyl sulfate, and carried out in a fume hood), gradually increased to 55 ° C for ten hours. After the reaction, it was cooled to room temperature, and the acetone was distilled by a rotary evaporator. Extract with ethyl acetate (2×100mL), wash with 1M hydrochloric acid (2×20mL), water (20mL) and saturated brine (20mL) successively, dry over anhydrous sodium sulf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com