A kind of allopurinol impurity C and preparation method thereof
A technology for allopurinol and impurities, which is applied in the field of compound preparation, can solve the problems of low content, low yield, and low purity, and achieve the effects of simple operation, simple preparation route, and increased electrophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] An allopurinol impurity C and a preparation method thereof, comprising the following steps:
[0031] S1: Weigh 5.0g 3-aminopyrazole-4-carboxamide hemisulfate, add 20g formamide to a 100ml reaction flask, add 1.2g hydrazine hydrate (80wt%) and 4g concentrated sulfuric acid (98wt%), Raise the temperature to 110°C and react for 8 hours;
[0032] S2: After the reaction of S1 is completed, cool the reaction liquid to room temperature, add 1mol / L sodium hydroxide solution to adjust the pH value to alkaline (pH value is about 14), add 50ml water and 50ml ethyl acetate, extract and separate the liquid, and collect the water phase ;
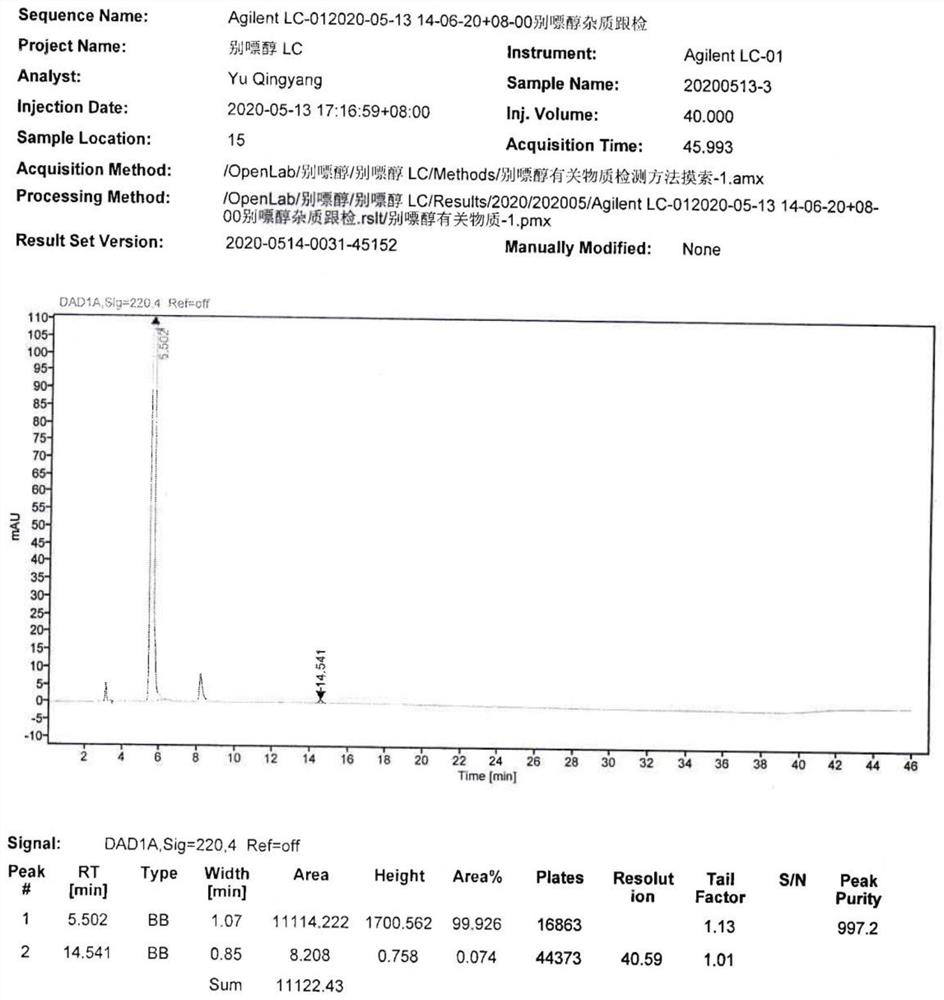
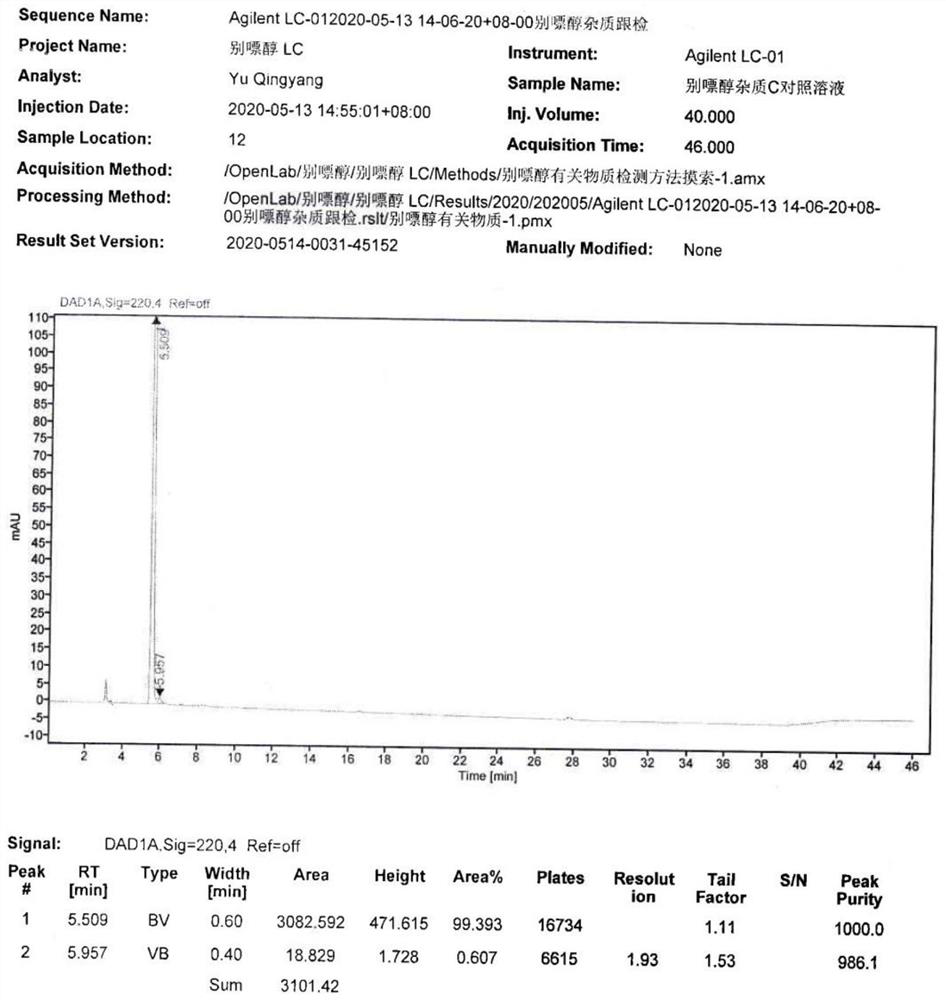
[0033] S3: Add 50ml of n-butanol to the water phase, extract and separate the liquid, collect the n-butanol phase, concentrate the n-butanol phase until a large amount of solids are precipitated, filter with suction, and vacuum-dry the filter cake at 60°C for 14 hours to obtain 4.41g of allopurinol impurity C, the yield is 86.7%, the purity is 99...
Embodiment 2
[0040] S1: Take 20.0g of 3-aminopyrazole-4-carboxamide hemisulfate, add it to a 500ml reaction flask containing 120g of formamide, add 5.2g of hydrazine hydrate (80wt%) and 18g of concentrated sulfuric acid (98wt%), and heat up To 120°C, react for 5h;
[0041] S2: After the reaction of S1 is completed, cool the reaction solution to room temperature, add 1mol / L sodium hydroxide solution to adjust to alkaline (pH value is 14), add 250ml of water and 250ml of ethyl acetate, extract and separate the liquid, and collect the water phase;
[0042] S3: Add 250ml of n-butanol to the water phase, extract and separate the liquid, collect the n-butanol phase, concentrate the n-butanol phase until a large amount of solids precipitate, filter with suction, and vacuum-dry the filter cake at 60°C for 13 hours to obtain 16.4g of allopurinol impurities C, the yield is 80.6%, and the purity is 99.839%.
Embodiment 3
[0044] S1: Get 1.0g 3-aminopyrazole-4-carboxamide hemisulfate, add in the 100ml reaction flask that 5g formamide is housed, add 0.25g hydrazine hydrate (80wt%) and 0.9g concentrated sulfuric acid (98wt%), Raise the temperature to 120°C and react for 6 hours;
[0045] S2: After the reaction of S1 is completed, cool the reaction solution to room temperature, add 1 mol / L sodium hydroxide solution to adjust to alkaline (pH about 14), add 50 ml of water and 50 ml of ethyl acetate, extract and separate the liquid, and collect the aqueous phase;
[0046] S3: Add 50ml of n-butanol to the water phase, extract and separate the liquid, collect the n-butanol phase, concentrate the n-butanol phase until a large amount of solids are precipitated, filter with suction, and dry the filter cake in vacuum at 60°C for 14 hours to obtain 0.86g of allopurinol Impurity C, the yield is 84.5%, and the purity is 99.841%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com