Engineered immune cells and uses thereof
An immune cell, engineering technology, applied in animal cells, genetically modified cells, cells modified by introducing foreign genetic material, etc., can solve the problem that CAR cells cannot infiltrate tumor tissue.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
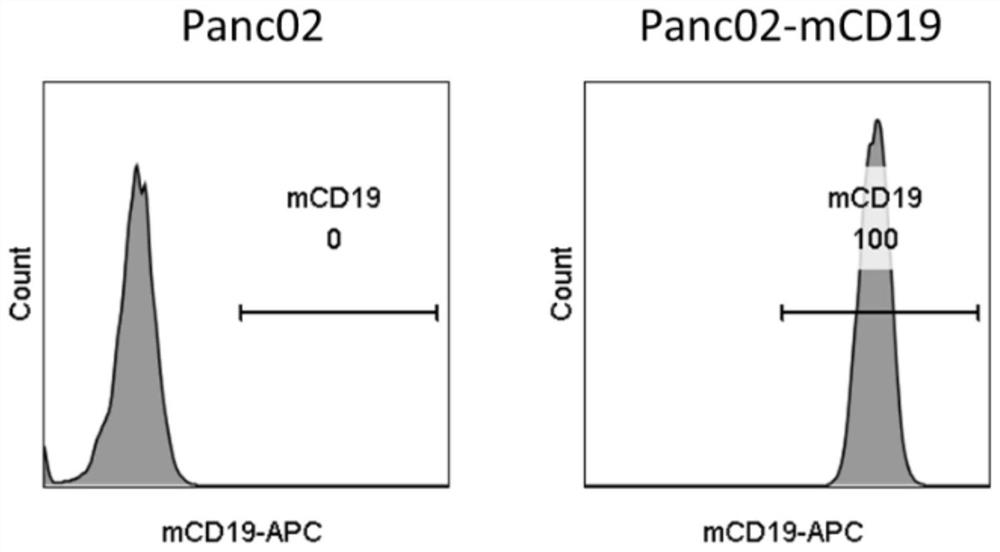
[0110] Example 1 Construction of mouse pancreatic cancer cell line Panc02-mCD19
[0111] 1. Preparation of pLV-mCD19 plasmid
[0112] Using the total mouse spleen mRNA as a template, the total cDNA sequence of the mouse spleen was obtained by reverse transcription PCR, and then the nucleic acid sequence of mCD19 (SEQ ID NO: 41) was obtained by PCR. Then, the mCD19 sequence was cloned into the pGEM-T Easy vector (Promega, Cat. No. A1360) to obtain the pLV-mCD19 plasmid.
[0113] 2. lentiviral packaging
[0114] In a T175 culture flask, 30×10 6 293T cells were inoculated in 30ml of DMEM medium containing 10% fetal calf serum, and cultured overnight in a 37°C, 5% CO2 incubator.
[0115] Add 3ml Opti-MEM (Gibco, Cat. No. 31985-070), 34 μg pLV-mCD19 plasmid, 8.5 μg pMD2.G vector (Addgene, Cat. No. 12259) and 17 μg psPAX2 vector (Addgene, Cat. No. 12260) into a sterile tube. Then add 120 μl X-treme GENE HP DNA transfection reagent (Roche, Cat. No. 06366236001), mix immediately,...
Embodiment 2
[0119] Example 2. Preparation of CAR-T cells
[0120] 1. Construction of Retroviral Plasmids
[0121] The DLL1 gene fragment (SEQ ID NO: 29) was artificially synthesized and recombined into the MSCV vector by Gibson to obtain the MSCV-DLL1 plasmid.
[0122] Artificially synthesized sequentially connected mCD19-scFv (SEQ ID NO: 13), CD8α hinge region (SEQ ID NO: 21), CD8α transmembrane region (SEQ ID NO: 15), 4-BB co-stimulatory domain (SEQ ID NO : 17) and the coding sequence fragment of CD3ζ intracellular domain (SEQ ID NO: 19), and XhoI / EcoRI restriction sites were added at both ends. The fragment was cloned into the MSCV vector to obtain the MSCV-mCD19-CAR plasmid.
[0123] Artificially synthesized sequentially connected mCD19-scFv (SEQ ID NO: 13), CD8a hinge region (SEQ ID NO: 21), CD8α transmembrane region (SEQ ID NO: 15), 4-BB co-stimulatory domain (SEQ ID NO : 17), CD3ζ intracellular domain (SEQ ID NO: 19), T2A (SEQ ID NO: 31) and Flt3L (SEQ ID NO: 27) coding sequence...
Embodiment 3
[0132] Example 3. Detecting the expression of CAR-T cells
[0133] 1. The expression level of CAR on the cell surface
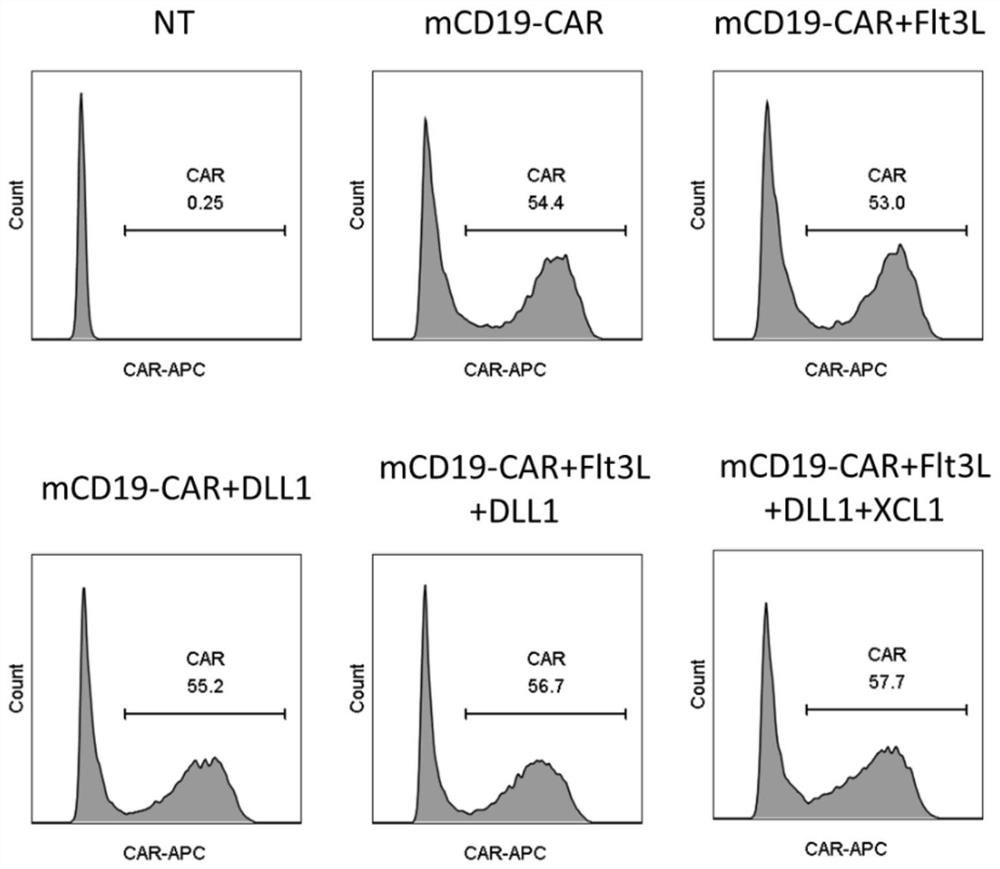
[0134] Take out the 2 * 10 that embodiment 2 prepares 5 CAR-T cells were detected by flow cytometry using Goat Anti-Rat IgG (H&L) Biotin (BioVision, Cat. No. 6910-250) as the primary antibody and APC Streptavidin (BD Pharmingen, Cat. No. 554067) as the secondary antibody The expression level of CAR on the cells, the results are as follows figure 2 shown.
[0135] It can be seen that the CAR positive efficiencies in mCD19-CAR, mCD19-CAR+Flt3L, mCD19-CAR+DLL1, mCD19-CAR+Flt3L+DLL1 and mCD19-CAR+Flt3L+DLL1+XCL1 cells compared with control NT cells Both were greater than 50%, indicating that these cells can effectively express CAR.
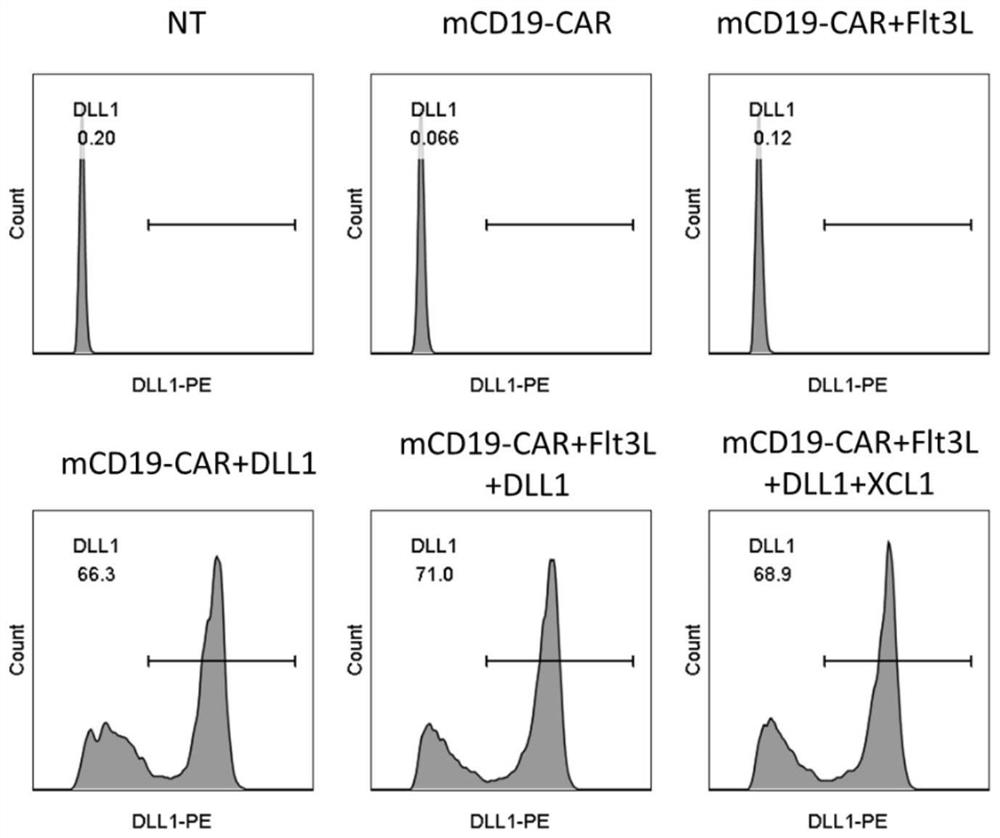
[0136] 2. Expression level of DLL1
[0137] Take out the 2 * 10 that embodiment 2 prepares 5 For each CAR-T cell, PE Anti-DLL1 antibody (Biolegend, catalog number 128307) was used to detect the expression level of DLL1 in CAR-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com