Preparation method of 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate
A technology of pentanediol monoisobutyrate and trimethyl is applied in the field of preparation of 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate and can solve the problem of decreasing catalytic activity , difficult distillation separation and other problems, to achieve the effect of less catalyst dosage, good selectivity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A preparation method of 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate, comprising the following steps:
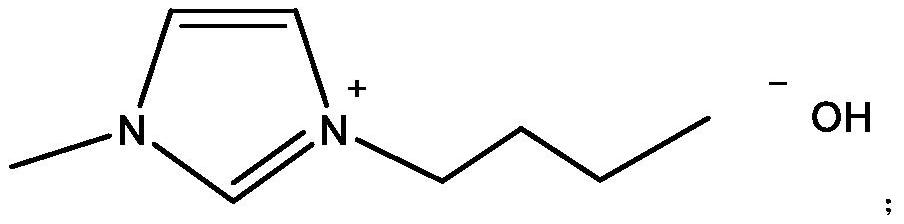
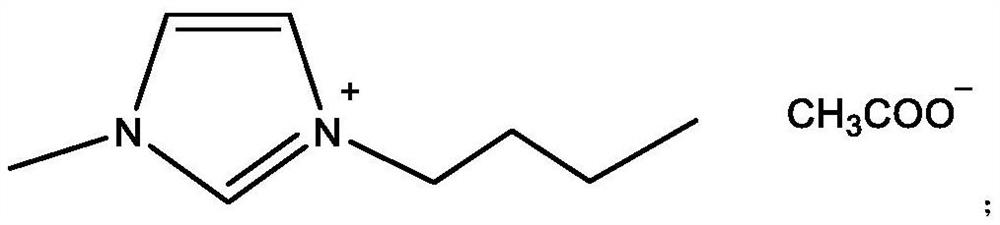
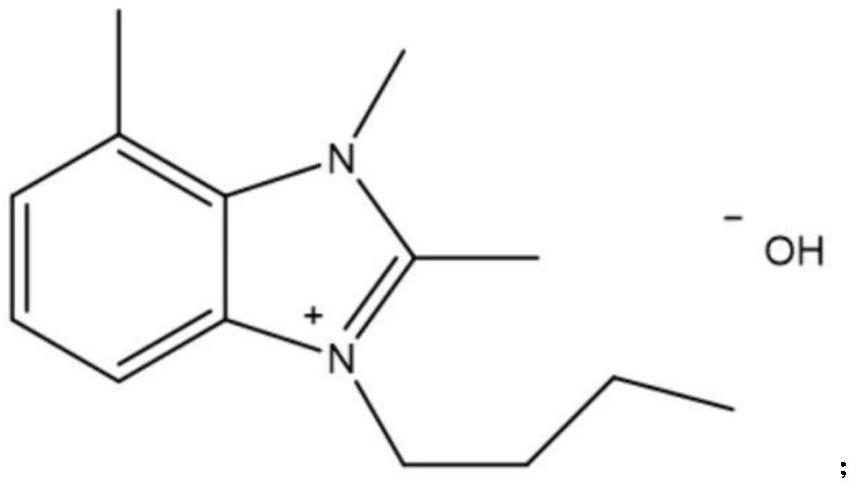
[0039] S1. In a 500ml three-neck flask equipped with a reflux condenser and a stirrer, add 100g isobutyraldehyde (1.389mol), and ionic liquid catalyst A, 10g, stir, and the reaction pressure is 0.5MPa;
[0040] S2. The temperature of the reaction system was raised to 65° C., and the stirring reaction was continued for 2 h.
[0041] S3. Cool the reaction system to room temperature, remove unreacted raw materials, and then separate by vacuum distillation to obtain 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate.
Embodiment 2
[0043] A preparation method of 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate, comprising the following steps:
[0044] S1. In a 500ml three-neck flask equipped with a reflux condenser and a stirrer, add 100g isobutyraldehyde (1.389mol), and ionic liquid catalyst B, 12.7g, stir, and the reaction pressure is 0.5MPa;
[0045] S2. The temperature of the reaction system was raised to 65° C., and the stirring reaction was continued for 2 h.
[0046] S3. Cool the reaction system to room temperature, remove unreacted raw materials, and then separate by vacuum distillation to obtain 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate.
Embodiment 3
[0048] A preparation method of 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate, comprising the following steps:
[0049] S1. In a 500ml three-neck flask equipped with a reflux condenser and a stirrer, add 100g isobutyraldehyde (1.389mol), and ionic liquid catalyst C, 20.3g, stir, and the reaction pressure is 0.5MPa;
[0050] S2. The temperature of the reaction system was raised to 65° C., and the stirring reaction was continued for 2 h.
[0051] S3. Cool the reaction system to room temperature, remove unreacted raw materials, and then separate by vacuum distillation to obtain 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com