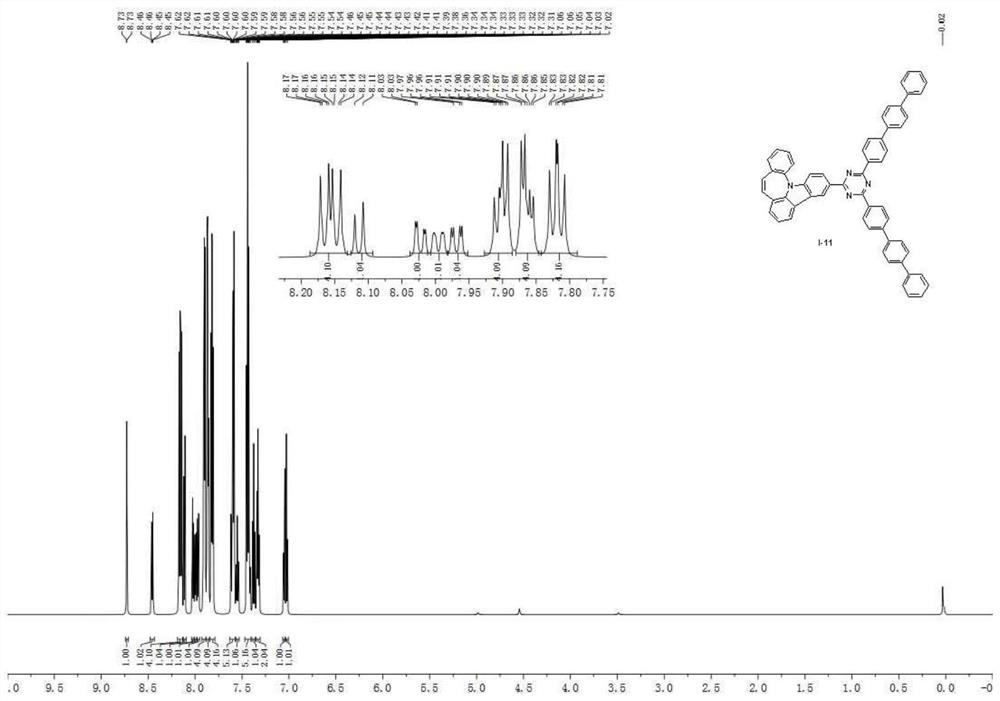
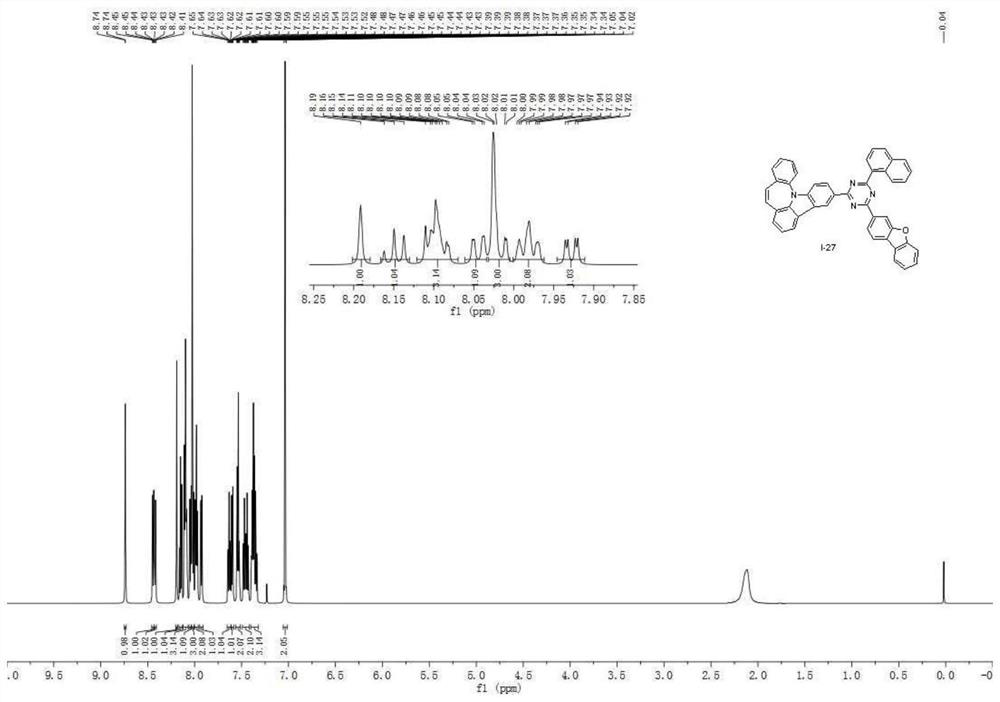
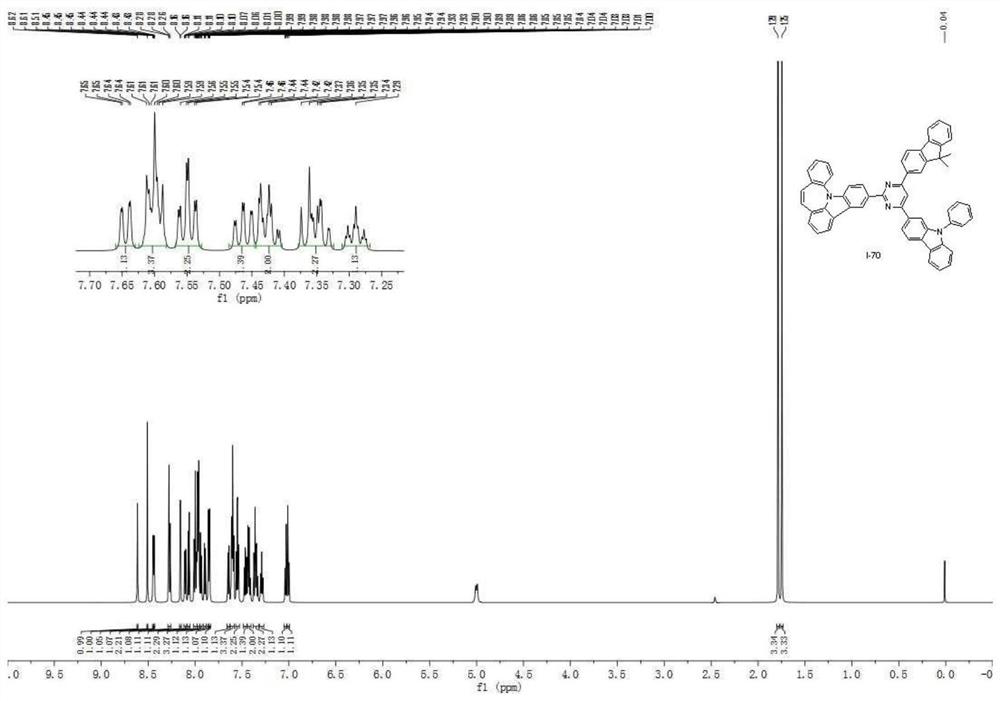
A seven-membered nitrogen-containing heterocyclic derivative and its organic electroluminescent device
A technology of derivatives and nitrogen heterocycles, which is applied in the field of seven-membered nitrogen-containing heterocycle derivatives and their organic electroluminescent devices, can solve the problems of device performance degradation, achieve reduced conjugation range, good film-forming properties, The effect of good performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The preparation of the seven-membered nitrogen-containing heterocyclic derivatives of the present invention, first, using iminostilbene (Y1) as a raw material, produces intermediate A through Buchwald-hartwing reaction with dibromoiodobenzene (X); then, the intermediate Body A is prepared by ring-forming reaction to obtain intermediate B; afterward, intermediate B reacts with biboronic acid pinacol ester to prepare intermediate C; finally, intermediate C and bromide Y2 occur Suzuki reaction to prepare target compound (I ), the specific synthetic route is as follows,
[0048]
[0049] Wherein, said L is selected from single bonds and arylene groups with 6 to 12 carbon atoms,
[0050] Said X 1 、X 2 、X 3 independently selected from CH or nitrogen atoms,
[0051] The Ar 1 、Ar 2 Independently selected from one of aryl groups with 6 to 30 carbon atoms and heteroaryl groups with 3 to 20 carbon atoms, the heteroaryl groups contain at least one of oxygen atom, sulfur at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com