Color microsphere immunochromatography test paper for rapidly diagnosing entamoeba histolytica antigen and preparation method thereof
A technology for immunochromatographic detection and amoeba, which is applied in the field of immunodiagnosis, can solve the problems of being unsuitable for primary detection, long detection time, and lack of specificity, and achieves reduced investment and detection costs, short detection time, and high specificity strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
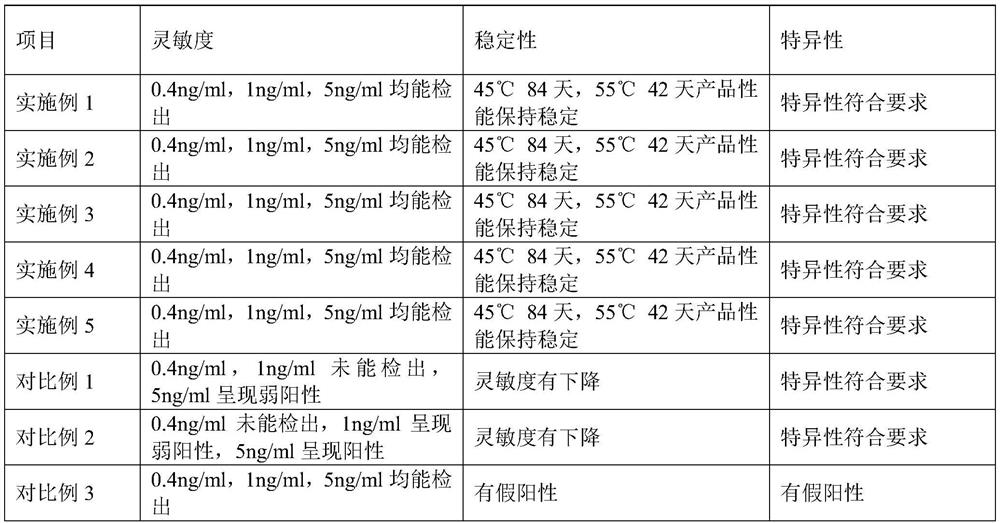
Examples
Embodiment 1
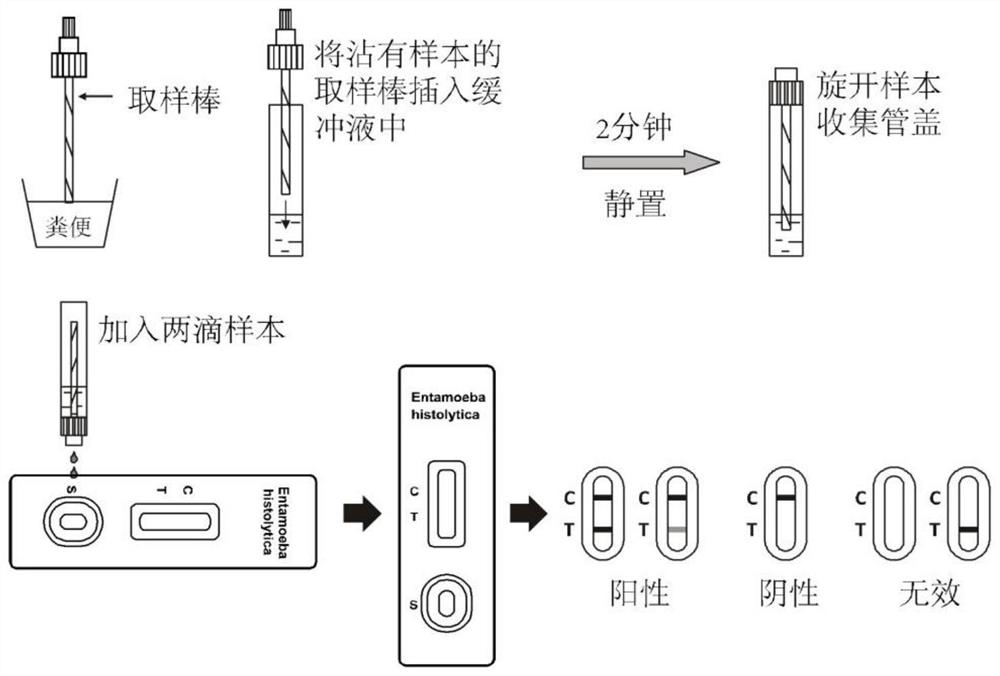
[0043] The preparation method of the color microsphere immunochromatography test paper for rapid diagnosis of Entamoeba histolytica specifically comprises the following steps:
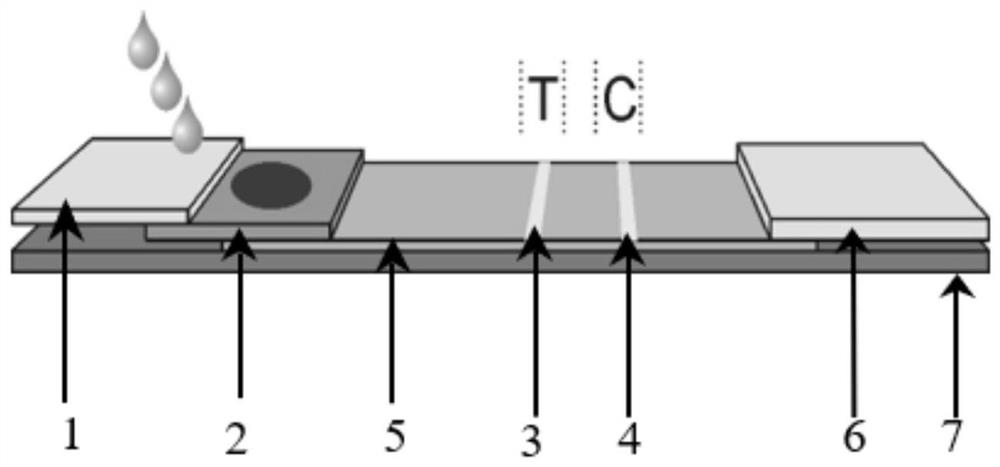
[0044] 1) Preparation of test line T and quality control line C: Dilute mouse anti-Entamoebic histolytica antibody and streptavidin-rabbit IgG conjugate solution respectively and spray them on nitric acid at a rate of 1.0ul / cm The cellulose membrane was used as the test line T and the quality control line C respectively, and dried at 36°C to obtain the test line and the quality control line; the test line T was coated with mouse anti-Entamoeba histolytica antibody The concentration is 0.1 mg / ml; the concentration of streptavidin-rabbit IgG conjugate coated with the quality control line C is 1.0 mg / ml;
[0045] 2) Preparation of the marker pad: when the diluted colored microsphere-antibody conjugate is at a concentration of 0.1%, it is treated on a polyester cellulose membrane at a rate of 2.0 ul / cm, an...
Embodiment 2
[0049] The difference from Example 1 is that the preparation method of the color microsphere immunochromatography test paper for rapid diagnosis of Entamoeba histolytica specifically includes the following steps:
[0050] 1) Preparation of test line T and quality control line C: Dilute mouse anti-Entamoebic histolytica antibody and streptavidin-rabbit IgG conjugate solution respectively and spray them on nitric acid at a rate of 1.05ul / cm The cellulose membrane was used as the test line T and the quality control line C respectively, and dried at 36.5°C to obtain the test line and the quality control line; the test line T was coated with mouse anti-Entamoeba histolytica antibody The concentration is 0.15 mg / ml; the concentration of streptavidin-rabbit IgG conjugate coated with the quality control line C is 1.2 mg / ml;
[0051] 2) Preparation of the marker pad: when the diluted colored microsphere-antibody conjugate was at a concentration of 0.15%, it was treated on a polyester c...
Embodiment 3
[0055] The difference from Example 1 is that the preparation method of the color microsphere immunochromatography test paper for rapid diagnosis of Entamoeba histolytica specifically includes the following steps:
[0056] 1) Preparation of test line T and quality control line C: Dilute mouse anti-Entamoebic histolytica antibody and streptavidin-rabbit IgG conjugate solution respectively and spray them on nitric acid at a rate of 1.1ul / cm The cellulose membrane was used as the test line T and the quality control line C respectively, and dried at 37°C to obtain the test line and the quality control line; the test line T was coated with mouse anti-Entamoeba histolytica antibody The concentration is 0.2 mg / ml; the concentration of streptavidin-rabbit IgG conjugate coated with the quality control line C is 1.5 mg / ml;
[0057] 2) Preparation of the marker pad: Dilute the colored microsphere-antibody conjugate to a concentration of 0.2%, process it on the polyester cellulose membrane...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com