Solid dispersion and pharmaceutical composition
A solid dispersion and composition technology, applied in the field of medicine, can solve problems such as poor bioavailability, low dissolution rate, and increased side effects of drugs, and achieve the effects of reducing clinical dosage, improving absorption properties, and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
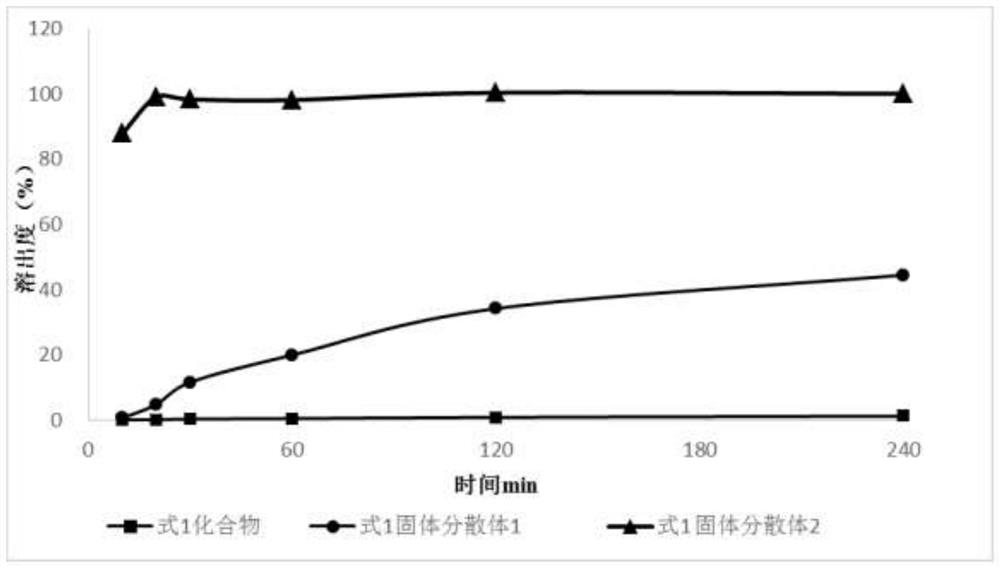
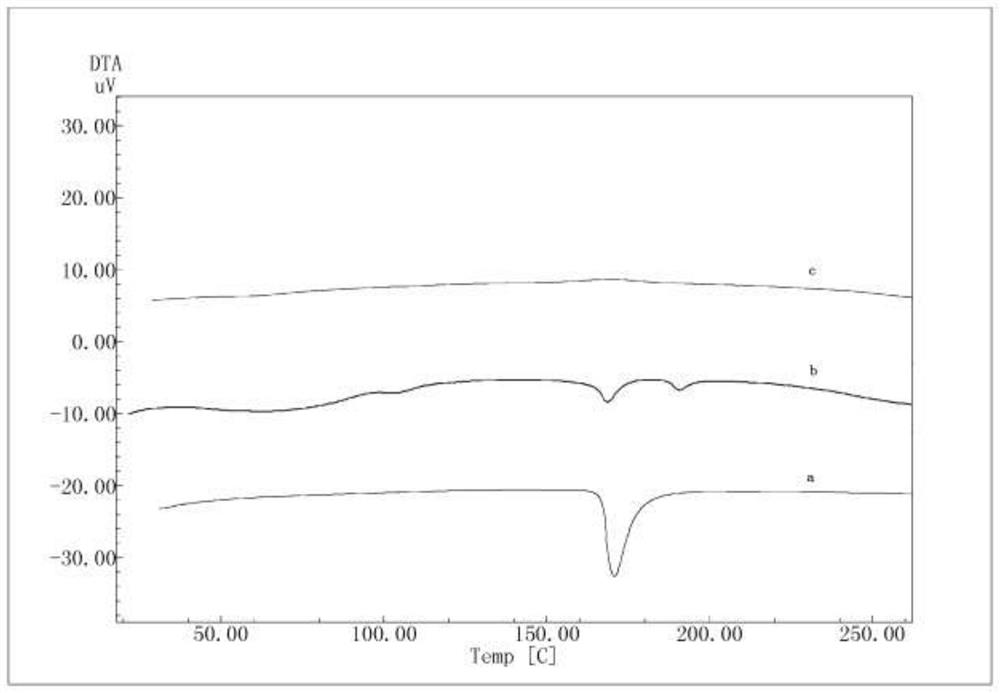
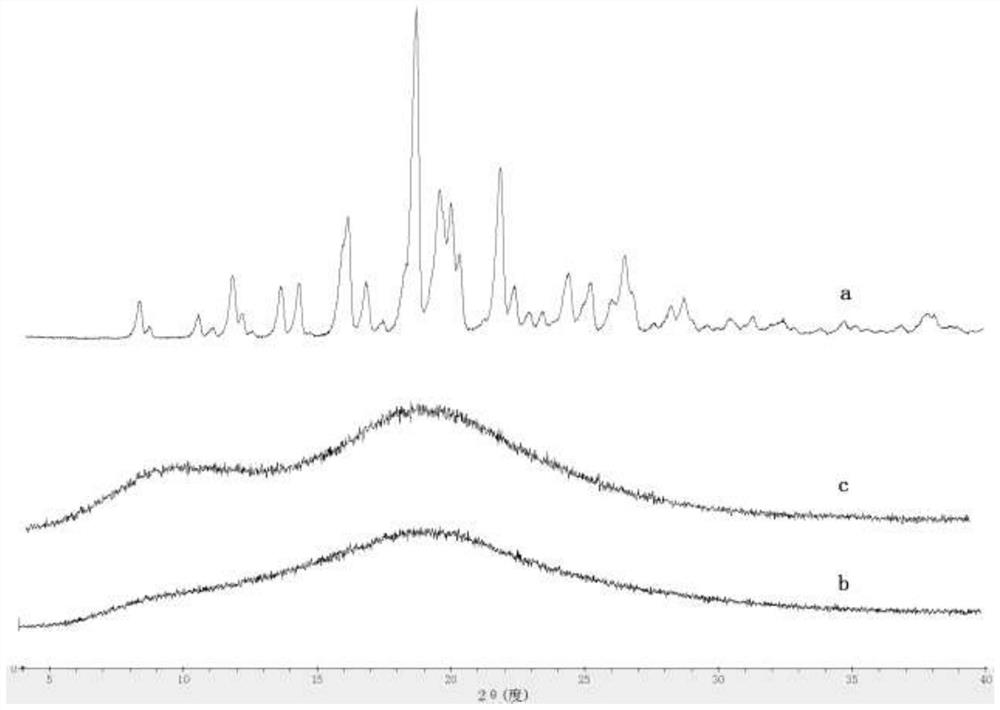
[0036] 1) Preparation of solid dispersion 1 of formula 1: add 1 g of compound of formula 1 to 100 mL of 95% ethanol, stir to dissolve to obtain a clear solution, then add 4 g Stir to dissolve; vacuum-dry at 40°C for 24 hours, pass through a 60-mesh sieve to obtain solid dispersion 1 (compound of formula 1+ ).
[0037] 2) Preparation of formula 1 solid dispersion 2: 1g of formula 1 compound is added in 100mL propanol, stirred to dissolve, and a clear solution is obtained; add 0.4g SDS, then add 4g Stir to make it uniform; vacuum-dry at 40°C for 24 hours, and pass through a 60-mesh sieve to obtain solid dispersion 2 (compound of formula 1+ +SDS).
[0038] 3) Formula 1 blend preparation: 1g formula 1 compound, 0.4g SDS, 4g Mix evenly to obtain a physical mixture (blend of formula 1).
Embodiment 2
[0040] Add 1g of the compound of formula 1 into 100mL of propanol, stir to dissolve, and obtain a clear solution; add 0.4g of SDS, and then add 3g Stir to make it uniform; remove the solvent by fluidized bed spray drying, and pass through a 60-mesh sieve to obtain a solid dispersion.
Embodiment 3
[0042] Add 1g of the compound of formula 1 to 15mL DMA, stir to dissolve, then add 4g HPMCAS, stir to dissolve to obtain an organic phase solution; under high shear conditions, slowly release the organic phase solution into 150ml purified water, filter, 40°C Vacuum-dried for 12 hours, and passed through an 80-mesh sieve to obtain a solid dispersion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| weight ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com