A kind of purification method of bendamustine hydrochloride
A technology of bendamustine hydrochloride and a purification method, which is applied in the field of purification of bendamustine hydrochloride, can solve the problems of poor product purity, difficulty in satisfying a single impurity, and inability to effectively remove impurities, etc., to achieve product The effect of good quality, short cycle and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
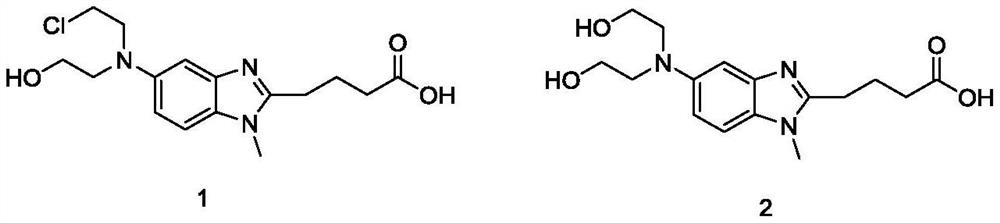
[0044] Place 15.0 g of 5-[bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butyric acid methyl ester in a three-necked reaction flask, add 150 mL of dichloromethane, drop 13.3g of thionyl chloride, and reacted at 20-30°C for 7 hours. Then add 75mL of concentrated hydrochloric acid dropwise, stir and separate at room temperature, retain the upper aqueous phase and raise the temperature to 60-70°C to continue the reaction for 12-18 hours, distill off the aqueous hydrochloric acid solution under reduced pressure, add 75mL of water and cool to 15-25°C to precipitate a solid. Add 2N NaOH aqueous solution dropwise to adjust the pH of the system to 1-2, then cool in an ice-water bath to 0-5°C, filter, rinse the filter cake with pre-cooled isopropanol and dry at 35°C for 8-12 hours to obtain 14.9g of hydrochloric acid Bendamustine crude.
Embodiment 2
[0046] Place 15.0 g of 5-[bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butyric acid methyl ester in a three-necked reaction flask, add 75 mL of dichloromethane, drop 16.0 g of thionyl chloride, and reacted at 20-30° C. for 7 hours. Then add 75mL of concentrated hydrochloric acid dropwise, stir and separate at room temperature, retain the upper aqueous phase and raise the temperature to 60-70°C to continue the reaction for 12-18 hours, distill off the aqueous hydrochloric acid solution under reduced pressure, add 75mL of water and cool to 15-25°C to precipitate a solid. Add 2N NaOH aqueous solution dropwise to adjust the pH of the system to 1-2, then cool in an ice-water bath to 0-5°C, filter, rinse the filter cake with pre-cooled isopropanol and dry at 35°C for 8-12 hours to obtain 15.5g of hydrochloric acid Bendamustine crude.
Embodiment 3
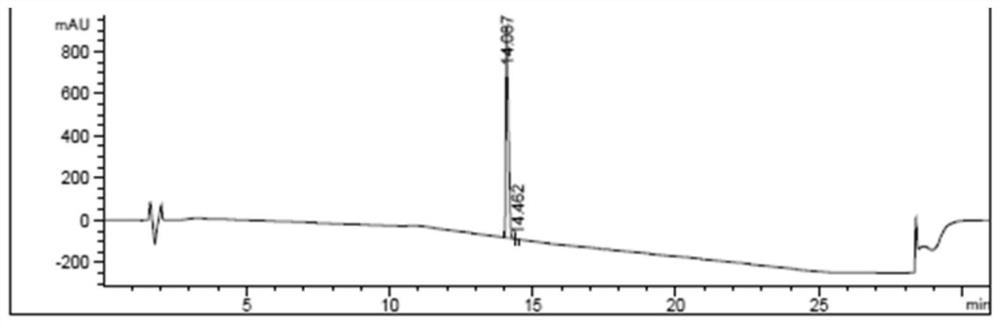
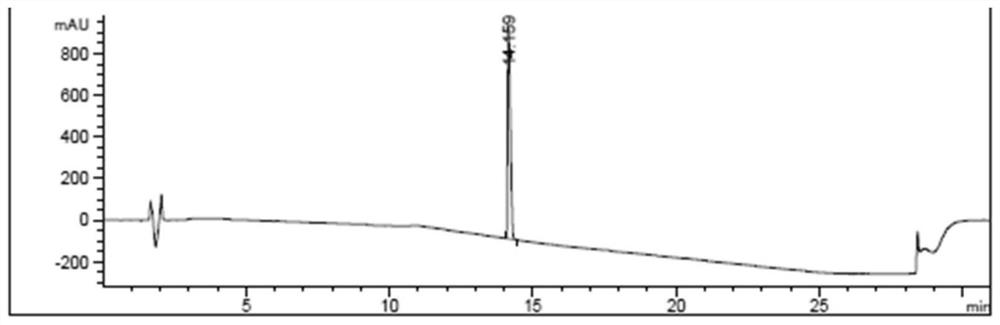
[0048]Put 6.4g of bendamustine hydrochloride crude product (containing HP1: 0.20%) in a three-necked reaction flask, add 32mL of 6mol / L hydrochloric acid aqueous solution, the temperature of the solution is 20-30°C, add 0.64g of activated carbon, stir and decolorize 2-3 hours, filter to remove activated carbon. Collect the mother liquor and add 10% Na dropwise 2 CO 3 The aqueous solution adjusts the pH of the system to 1-2, and then cools in an ice-water bath to 0-5°C for recrystallization. After filtering, the filter cake was rinsed with pre-cooled acetone and dried at 35° C. for 16 hours to obtain 5.9 g of bendamustine hydrochloride. Yield of bendamustine hydrochloride: 92%; HPLC purity: 99.94%, HP1: not detected, single impurity: 0.06%, see attached figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com