Synthetic method of chromanone compounds
A synthetic method and compound technology, which is applied in the field of synthesis of chromanone compounds, can solve the problems of not being chromanone compounds, uneconomical, and unfriendly to the environment, and achieve simple reaction system, effective recycling, and short reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The embodiment of the present invention provides a synthetic method of chromanone compounds, comprising the following steps:
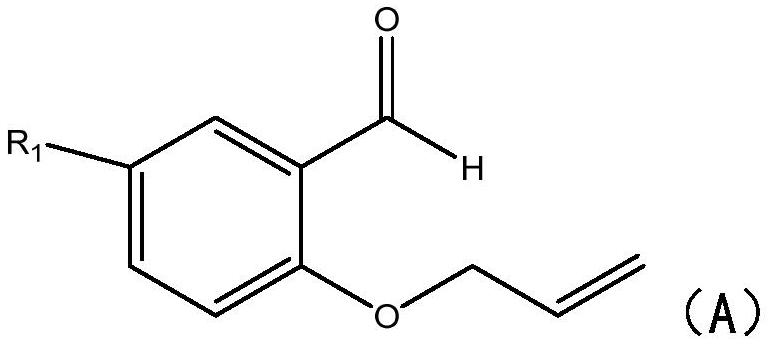
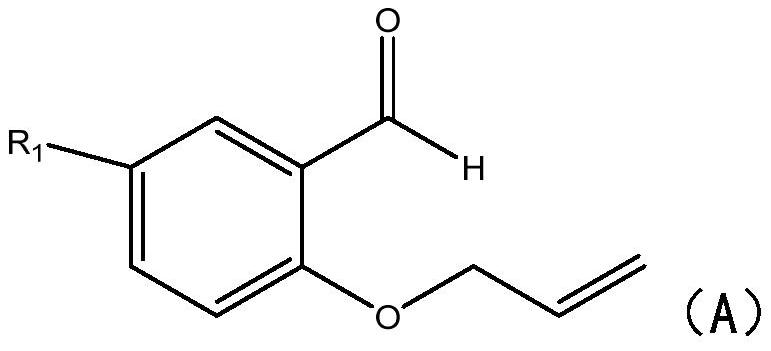
[0024] S1: Add 2-(allyloxy)benzaldehyde compounds and sodium benzenesulfinate compounds to the reactor respectively, under the action of a catalyst and a deep eutectic solvent, react in an airtight condition at 60-100°C 5-8 hours.
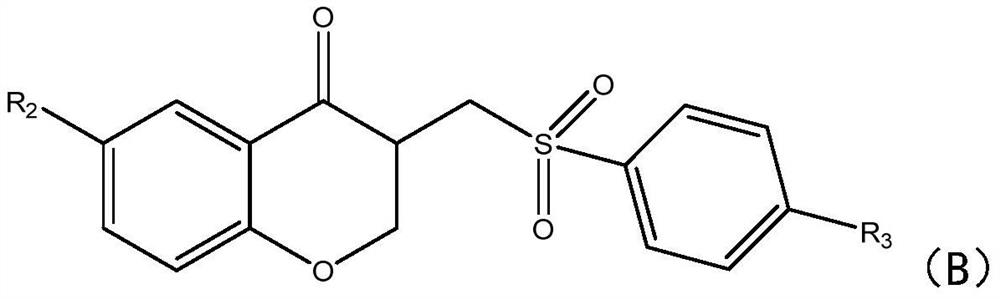
[0025] In this step, 2-(allyloxy)benzaldehyde compounds and sodium benzenesulfinate compounds are used to synthesize chromanone compounds, specifically, under heating conditions, ferric chloride and persulfate Under the action of the deep eutectic solvent, the hydrogen atom of the sodium benzene sulfinate compound is captured to generate a sulfinic acid free radical, and the sulfinic acid free radical passes through the double bond in the 2-(allyloxy)benzaldehyde compound Addition generates carbon free radicals, which then undergo intramolecular cyclization reactions with aldehyde groups to generate oxygen free radi...
Embodiment 1
[0044] Add 1mmol to the reactor 1mmol sodium benzenesulfinate, 2mmol potassium persulfate, 0.05mmol FeCl 3 ·6H 2 O and 2ml of deep eutectic solvent, under air condition of 80 ℃ airtight heat reaction 6 hours;
[0045]
[0046] Carry out nuclear magnetic spectrum analysis to above-mentioned white solid powder, data is as follows:
[0047] 1 H NMR (500MHz, CDCl 3 )δ8.03-7.95(m,2H),7.83(dd,J=7.9,1.6Hz,1H),7.69(t,J=7.5Hz,1H),7.61(t,J=7.7Hz,2H), 7.54-7.47(m,1H),7.01(dd,J=15.7,7.8Hz,2H),5.01(dd,J=11.4,5.3Hz,1H),4.35(t,J=11.8Hz,1H),3.99 (dd, J=14.6, 2.3Hz, 1H), 3.51-3.36(m, 1H), 3.01(dd, J=14.6, 10.1Hz, 1H);
[0048] 13 C NMR (126MHz, CDCl 3 )δ189.9, 161.7, 138.9, 136.60, 134.2, 129.6, 128.0, 127.6, 121.8, 119.9, 118.0, 69.62, 51.75, 40.8;
[0049] After identification, the spectral data corresponds to the structural formula, proving that the synthesized product is 3-((benzenesulfonyl)methyl)benzopyran-4-one with a yield of 90%.
Embodiment 2
[0051] Add 1mmol to the reactor 1mmol sodium p-chlorobenzenesulfinate, 2mmol potassium persulfate, 0.05mmol FeCl 3 ·6H 2 O and 2ml of deep eutectic solvent, under air condition of 60 ℃ of airtight heating reaction for 8 hours; After the reaction is finished, carry out column chromatography separation, obtain following P2 compound:
[0052]
[0053] Carry out nuclear magnetic spectrum analysis to above-mentioned white solid powder, data is as follows:
[0054] 1 H NMR (500MHz, CDCl 3 )δ7.91(d, J=7.7Hz, 2H), 7.83(d, J=7.9Hz, 1H), 7.58(d, J=7.6Hz, 2H), 7.51(t, J=7.8Hz, 1H) ,7.02(dd,J=18.6,7.9Hz,2H),5.00(dd,J=11.4,5.2Hz,1H),4.35(t,J=11.8Hz,1H),3.97(d,J=14.6Hz, 1H), 3.50-3.32(m, 1H), 3.01(dd, J=14.3, 10.2Hz, 1H);
[0055] 13 C NMR (126MHz, CDCl 3 )δ189.8, 161.7, 141.1, 137.3, 136.7, 129.9, 129.5, 127.6, 121.8, 119.9, 118.1, 69.6, 51.8, 40.8;
[0056] After identification, the spectrum data corresponds to the structural formula, proving that the synthesized product is 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com