Cyclodextrin hyperbranched derivative and preparation method thereof
A technology of cyclodextrin and derivatives, applied in the field of cyclodextrin hyperbranched derivatives and their preparation, can solve the problems of β-cyclodextrin derivatives cationic loss, limited number of hydroxyl values, insufficient hydrophilicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] In this embodiment, a cyclodextrin derivative and a preparation method thereof comprise the following steps:
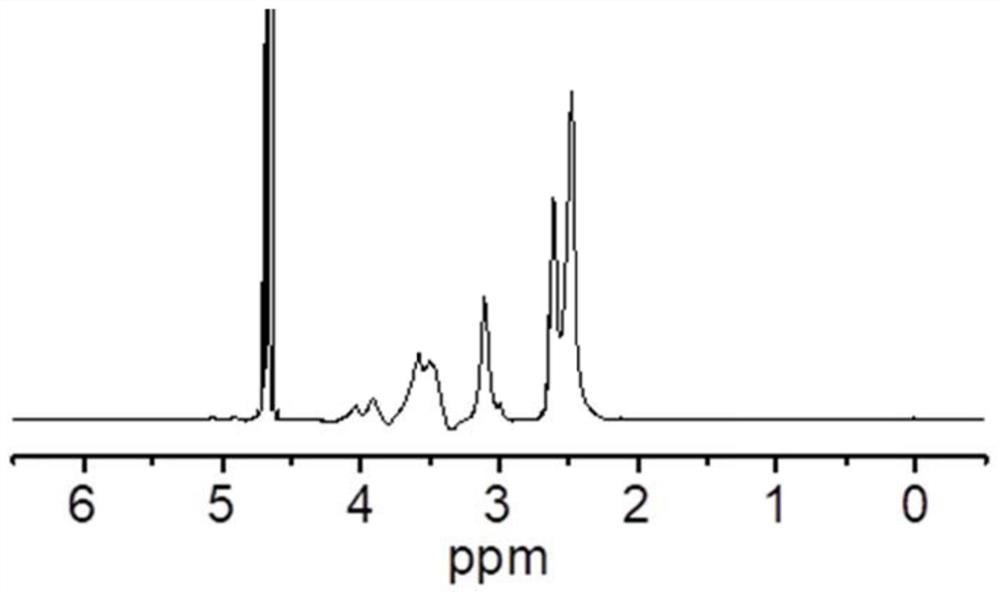
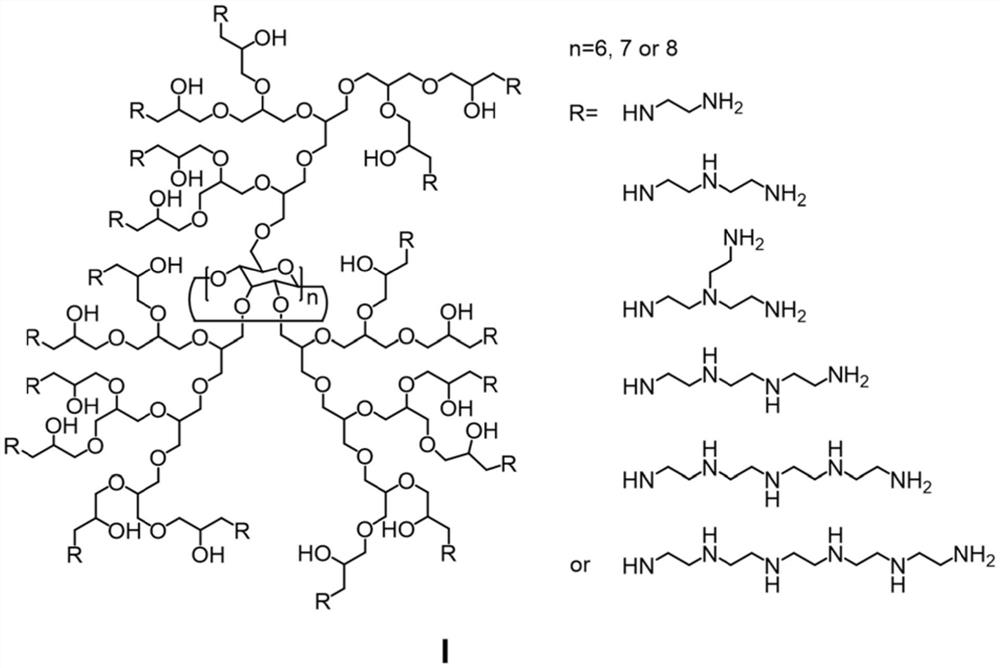
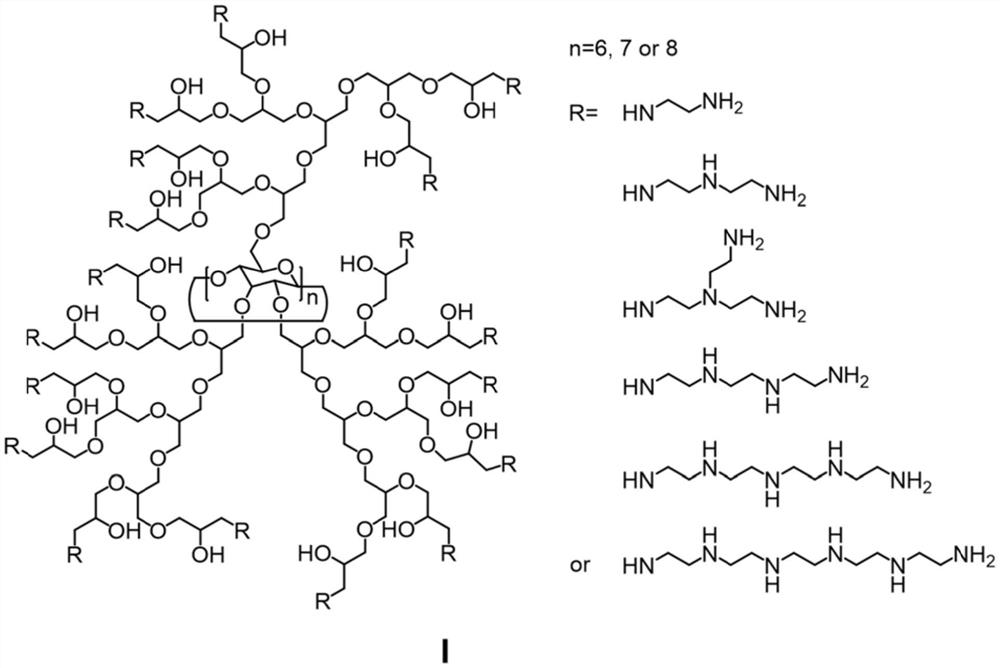
[0047](1) Accurately weigh β-cyclodextrin (4.0g, 3.53mmol) and 18-crown-6 (6.6g, 25.0mmol) under inert gas protection conditions, dissolve in 150mL DMF, and then add KH (1.12g , 28.0mmol) and keep stirring to fully react with the hydroxyl group of cyclodextrin. The temperature was controlled at 50°C, and glycidol (15.0 g, 202.6 mmol) dissolved in 100 mL of DMF was slowly added dropwise to the above solution for about 24 hours. Control the temperature at 80°C and continue the reaction for 16h to end. After the temperature of the system was lowered to room temperature, a small amount of water was added to terminate the reaction, and the water was directly dialyzed (MWCO: 1000) and freeze-dried to obtain 17.1 g of β-cyclodextrin grafted hyperbranched polyglycerol;
[0048] (2) Take the β-cyclodextrin grafted hyperbranched polyglycerol obtained in the previous st...
Embodiment 2
[0051] In this embodiment, a cyclodextrin derivative and a preparation method thereof comprise the following steps:
[0052] (1) Accurately weigh β-cyclodextrin (4.0g, 3.53mmol) and 18-crown-6 (6.6g, 25.0mmol) under inert gas protection conditions, dissolve in 150mL DMF, and then add KH (1.12g , 28.0mmol) and keep stirring to fully react with the hydroxyl group of cyclodextrin. The temperature was controlled at 50°C, and glycidol (30.0 g, 405.2 mmol) dissolved in 100 mL of DMF was slowly added dropwise to the above solution for about 24 hours. Control the temperature at 80°C and continue the reaction for 16h to end. After the temperature of the system was lowered to room temperature, a small amount of water was added to terminate the reaction, and the water was directly dialyzed (MWCO: 1000) and freeze-dried to obtain 32.3g of β-cyclodextrin grafted hyperbranched polyglycerol;
[0053] (2) Take the β-cyclodextrin grafted hyperbranched polyglycerol obtained in the previous st...
Embodiment 3
[0056] In this embodiment, a cyclodextrin derivative and a preparation method thereof comprise the following steps:
[0057] (1) Accurately weigh α-cyclodextrin (3.43g, 3.03mmol) and 18-crown-6 (6.6g, 25.0mmol) under inert gas protection conditions, dissolve in 150mL DMF, and then add KH (1.12g , 28.0mmol) and keep stirring to fully react with the hydroxyl group of cyclodextrin. The temperature was controlled at 50°C, and glycidol (15.0 g, 202.6 mmol) dissolved in 100 mL of DMF was slowly added dropwise to the above solution for about 24 hours. Control the temperature at 80°C and continue the reaction for 16h to end. After the temperature of the system was lowered to room temperature, a small amount of water was added to terminate the reaction, and the water was directly dialyzed (MWCO: 1000) and freeze-dried to obtain 16.5g of β-cyclodextrin grafted hyperbranched polyglycerol;
[0058] (2) Disperse the α-cyclodextrin grafted hyperbranched polyglycerol obtained in the previo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com