Mutant homoserine kinase and application thereof
A technology of homoserine kinase and aspartate kinase, which is applied in the field of amino acid modification, can solve problems such as difficulty in feedback inhibition, and achieve the effects of releasing feedback inhibition, improving production capacity and improving feedback inhibition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Screen the anti-AHV microorganism by artificial modification
[0035] In this embodiment, use Corynebacterium glutamicum ATCC14067 as parent bacterial strain, use NTG (with N-methyl-N'-nitro-N-nitrosoguanidine) to bacterial strain ATCC14067 to carry out mutagenesis screening anti-AHV (2 -amino-3-hydroxyvaleric acid, a threonine analog) strain.
[0036] The mutagenesis process is to transfer the ATCC14067 bacterial strain cultivated in the seed medium for 15h to 5ml of the seed medium and cultivate it to OD 560 to 1.7. Centrifuge the medium to recover the cells, then wash the cells twice with 80mM citrate buffer, then suspend the cells with 5ml of the same buffer solution, and add NTG with a final concentration of 200ug / ml for 20min. The resultant was then washed twice with the same buffer. Finally, they were respectively coated on basic medium containing AHV with final concentrations of 20mM, 40mM and 50mM, and cultured for 2-4 days to obtain variants w...
Embodiment 2
[0039] Example 2: L-threonine production test for AHV-resistant strains
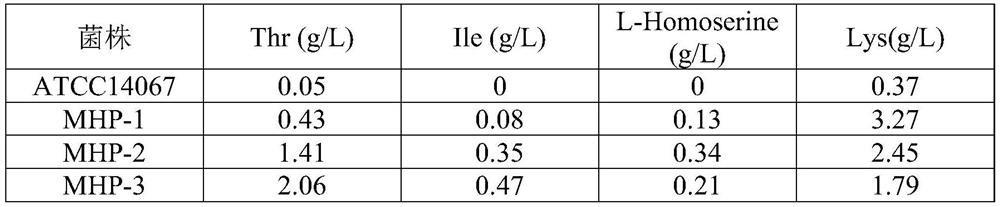
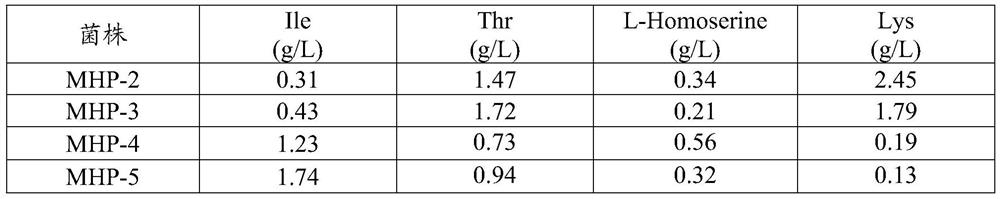
[0040] 87 mutant strains were obtained by the method in Example 1, and the threonine production capacity test was performed on the obtained 87 mutant strains. Inoculate 87 strains of mutant strains and control strain Corynebacterium glutamicum ATCC14067 into an Erlenmeyer flask (500ml) containing 25ml of seed medium, then shake and culture at 110rpm at 30-31°C for 15-17h to an OD value of 16- 18. Transfer 10% of the inoculum to a 25 ml Erlenmeyer flask (500 ml) containing a threonine production medium, and then culture at 30-31° C. with shaking at 135 rpm for 48 hours. After cultivation, HPLC was used to detect the content of various amino acids. Table 1 shows the concentrations of main amino acids in the fermentation liquid of 11 strains showing excellent L-threonine production ability among 87 strains. The above 11 strains were named SMI001 to SMI011. As shown in Table 2, among the 11 strains, SMI00...
Embodiment 3
[0044] Example 3: Analysis of the nucleotide sequence of the gene thrB derived from the strain with the ability to produce L-threonine derived from SMI001
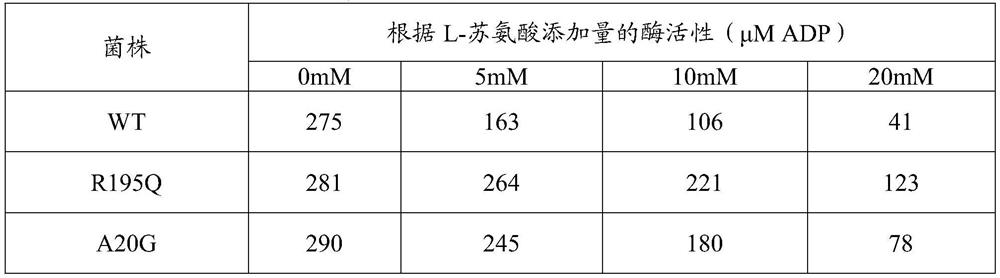
[0045] This example attempts to confirm whether the mutation of gene thrB encoding homoserine kinase (HK) occurs in the variant SMI001. Referring to the sequence of the thrB gene of NCBI strain 14067 (shown in SEQ ID NO: 1), primers were designed to amplify the chromosomal DNA of the variant using the polymerase chain reaction (hereinafter referred to as "PCR") method. Specifically, the genome of the variant was used as a template and primers PI-thrB-F and PI-thrB-R and PfuUltraTM high-fidelity DNA polymerase were used as polymerases for PCR reactions. PCR conditions were as follows: denaturation at 95°C for 30 seconds; annealing at 52°C for 20 seconds; and polymerization at 72°C for 2 minutes, and repeated for a total of 30 cycles. Result: A gene fragment of 1471 bp can be amplified, which includes 129 bp upstream of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com