A kind of synthetic method of pregabalin intermediate
A technology of pregabalin and synthesis method, which is applied in the directions of botanical equipment and methods, biochemical equipment and methods, enzymes, etc., can solve the problems of poor chiral selection of products, low transformation efficiency and high cost, and achieves simple operation and transformation. High rate and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] This embodiment provides a hydantoinase, the nucleotide sequence of which is shown in SEQ ID NO: 1 in the Sequence Listing, and the amino acid sequence is shown in SEQ ID NO: 2 in the Sequence Listing. Concretely, the preparation method of this hydantoin enzyme comprises the following steps:
[0022] S1. Take the existing commercially available pACYCDuet vector as the expression vector.
[0023] S2. After digesting the pACYCDuet vector, insert the nucleotide sequence of hydantoinase shown in the sequence table SEQ ID NO:1 between the NdeI and AvrII restriction sites of the digested pACYCDuet vector to obtain Recombinant expression vector.
[0024] S3, the above-mentioned recombinant expression vector is transformed into BL21 (DE3) escherichia coli, obtains bacterium colony; Then, pick single bacterium colony and inoculate in the 250mL conical flask containing the LB medium of 50mL with inoculating loop, place conical flask in Shaking incubator, 30 ℃, 250rpm cultivated...
Embodiment 2
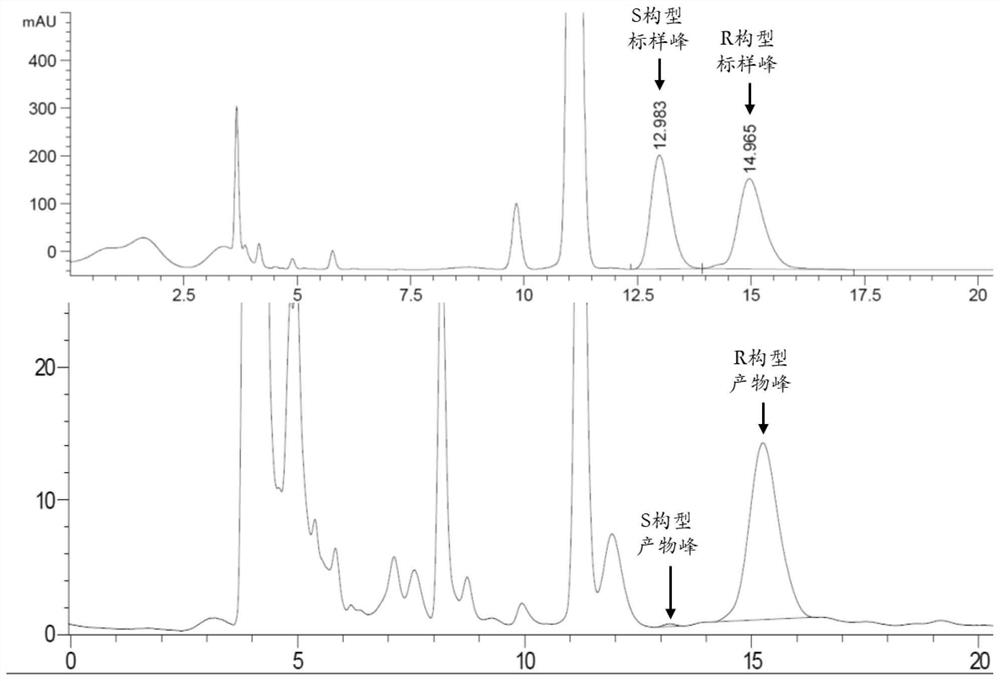
[0026] This embodiment provides a kind of synthetic method of pregabalin intermediate, and the chemical name of this pregabalin intermediate is (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid , the synthetic route of this synthetic method is as follows:
[0027]
[0028] Specifically, the synthesis method comprises the following steps:
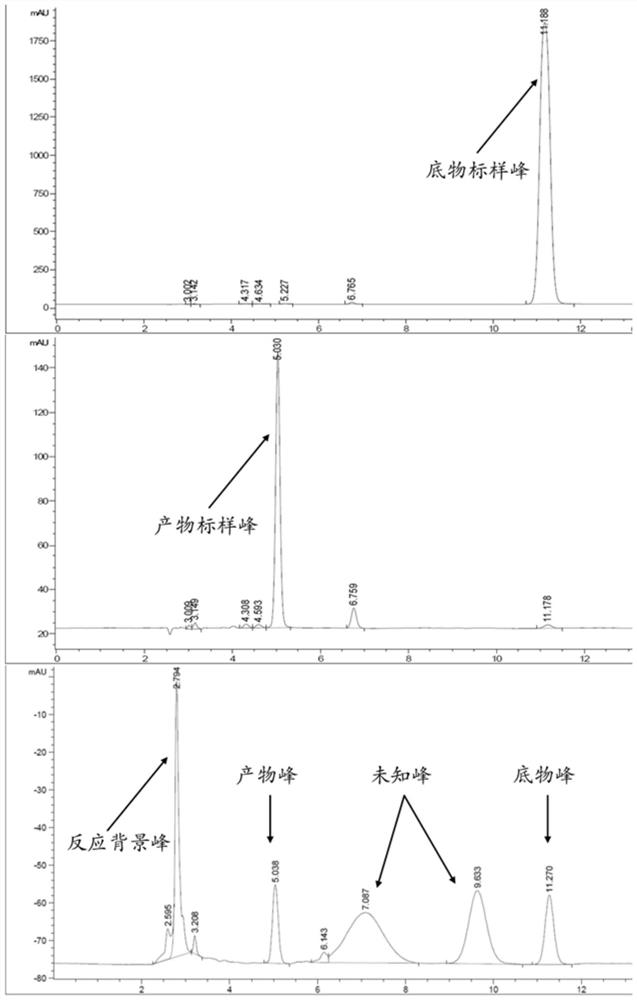
[0029] S1. Place 0.01 g of 3-(2-methylpropyl) glutarimide and 0.25 g of the Escherichia coli wet cells containing hydantoinase obtained in Example 1 above into 5 mL of pH 7.5 The enzymatic reaction was carried out in a reactor of 50 mM aqueous phosphate buffer solution for 48 hours, and the temperature of the buffer solution in the reactor was controlled at 30° C. to obtain a reaction solution.
[0030] S2. Add 4 times the volume of acetonitrile to the above reaction solution for vibration inactivation, then centrifuge at 13000rmp for 3 minutes, and take the supernatant to obtain the reaction product containing the pregabalin intermediat...
Embodiment 3
[0032] This embodiment provides a kind of synthetic method of pregabalin intermediate, and the chemical name of this pregabalin intermediate is (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid , the synthesis method comprises the following steps:
[0033] S1. Place 0.01 g of 3-(2-methylpropyl) glutarimide and 0.2 g of E. coli wet cells containing hydantoinase obtained in Example 1 above into 5 mL of pH 6 The enzymatic reaction was carried out in a reactor of aqueous phosphate buffer solution for 48 hours, and the temperature of the buffer solution in the reactor was controlled at 20° C. to obtain a reaction solution.
[0034] S2. Add 4 times the volume of acetonitrile to the above reaction solution for vibration inactivation, then centrifuge at 12000rmp for 3 minutes, and take the supernatant to obtain the reaction product containing the pregabalin intermediate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com