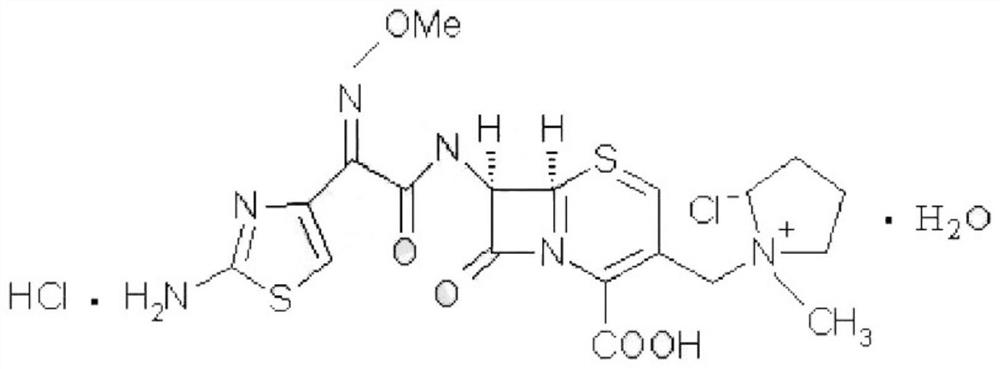
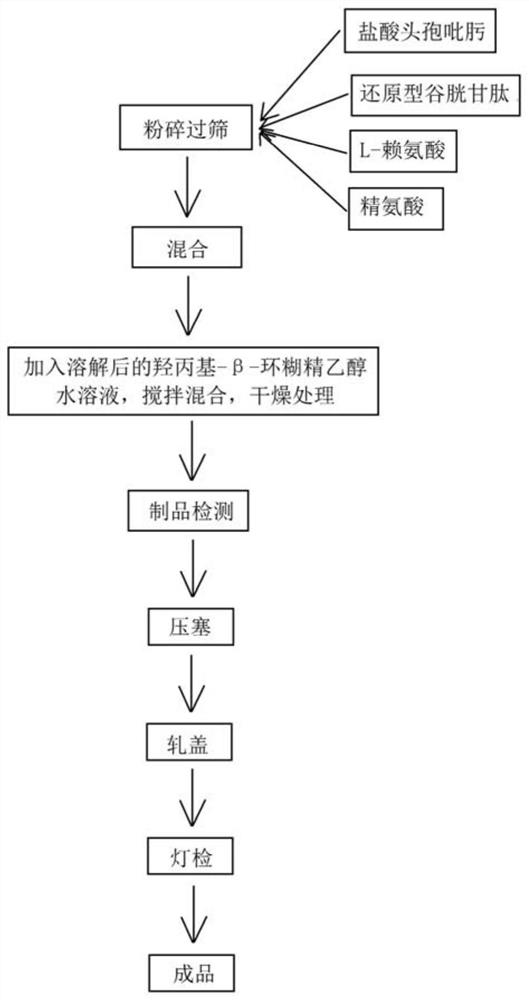
Cefepime hydrochloride for injection and preparation method of cefepime hydrochloride
A technology for cefepime hydrochloride and injection, which is applied in the field of medicinal chemistry, can solve the problems of uneven packaging of cefepime hydrochloride powder, different electrostatic adsorption amounts, and large differences in bulk density, and achieve good packaging uniformity, The effect of improving purity and content and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
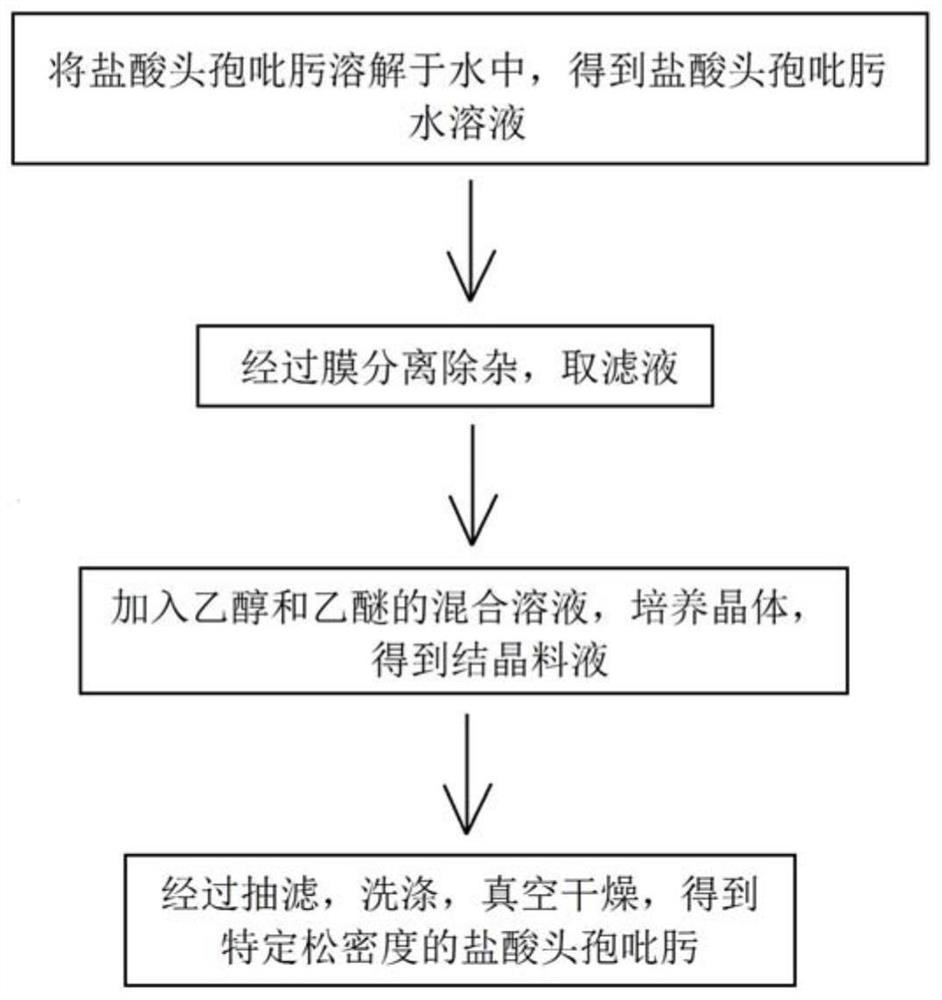
[0032] The preparation of cefepime hydrochloride, such as figure 2 shown, including the following steps:
[0033] (1) Dissolving 50 g of cefepime hydrochloride in 1500 mL of water under stirring conditions at 50-60° C. to obtain an aqueous solution of cefepime hydrochloride;
[0034] (2) the aqueous solution of cefepime hydrochloride prepared in step (1) is carried out membrane separation and impurity removal through a polysulfone membrane with a molecular weight cut-off of 6000D and a polysulfone membrane with a molecular weight cut-off of 2000D under an operating pressure of 0.5MPa, and takes the filtrate;
[0035] (3) Adding a mixed solution of ethanol and ether with a volume ratio of 1:1 to the filtrate prepared in step (2), cooling the temperature to 0-5° C. in an ice-water bath, and cultivating crystals under stirring conditions to obtain a crystallization feed liquid;
[0036] (4) The crystalline liquid obtained in step (3) is filtered, washed, and vacuum-dried to obt...
Embodiment 2
[0043] The preparation of cefepime hydrochloride:
[0044] (1) Dissolving 50 g of cefepime hydrochloride in 2000 mL of water under stirring conditions at 50-60° C. to obtain an aqueous solution of cefepime hydrochloride;
[0045] (2) the cefepime hydrochloride aqueous solution prepared by step (1) is carried out membrane separation and impurity removal through the polysulfone membrane with a molecular weight cut-off of 6000D and the polysulfone membrane with a molecular weight cut-off of 2000D under 0.4MPa operating pressure successively, and takes the filtrate;
[0046] (3) Adding a mixed solution of ethanol and ether with a volume ratio of 1:1 to the filtrate prepared in step (2), cooling the temperature to 0-5° C. in an ice-water bath, and cultivating crystals under stirring conditions to obtain a crystallization feed liquid;
[0047] (4) The crystal feed liquid obtained in step (3) is filtered, washed, and vacuum-dried to obtain a bulk density of 0.42 g / cm 3 of cefepime h...
Embodiment 3
[0054] The preparation of cefepime hydrochloride:
[0055] (1) Dissolving 50 g of cefepime hydrochloride in 1800 mL of water under stirring conditions at 50-60° C. to obtain an aqueous solution of cefepime hydrochloride;
[0056] (2) The cefepime hydrochloride aqueous solution prepared in step (1) is subjected to membrane separation and impurity removal through a sulfonated polysulfone membrane with a molecular weight cut-off of 6000 D and a sulfonated polysulfone membrane with a molecular weight cut-off of 2000 D under an operating pressure of 0.5 MPa , take the filtrate;
[0057] (3) Adding a mixed solution of ethanol and ether with a volume ratio of 1:1 to the filtrate prepared in step (2), cooling the temperature to 0-5° C. in an ice-water bath, and cultivating crystals under stirring conditions to obtain a crystallization feed liquid;
[0058] (4) The crystal feed liquid obtained in step (3) is filtered, washed, and vacuum-dried to obtain a bulk density of 0.53 g / cm 3 o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com