Red phosphor and preparation method and application of red phosphor
A technology of red fluorescent powder and fluorescent powder, which is applied in the direction of chemical instruments and methods, luminescent materials, etc., can solve the problems of no Mn transfer characteristics, fluorescent powder is difficult to dissolve in water, etc., and achieve improved luminous intensity, simple method, and high luminous intensity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A preparation method of fluoride-based fluorescent powder, carried out as follows:
[0046] (1) Preparation of KHF 2 :Mn 4+ : Add KF to KMnO 4 React directly with the mixed solution formed by HF with a mass concentration of 26% at normal temperature and pressure, filter, wash and dry after the reaction, KF, HF and KMnO 4 The dosage relationship is 150:250:6;
[0047] (2) Mn 4+ Transfer: KHF 2 :Mn 4+ Dissolve in solvent distilled water, add fluoride matrix K 2 TiF 6 Place the reaction, wash, filter and dry after the reaction to obtain new doped Mn 4+ The red phosphor product K 2 TiF 6 :Mn 4+ , KHF 2 :Mn 4 + 、K 2 TiF 6 The molar ratio of matrix and distilled water: 1:4:80.
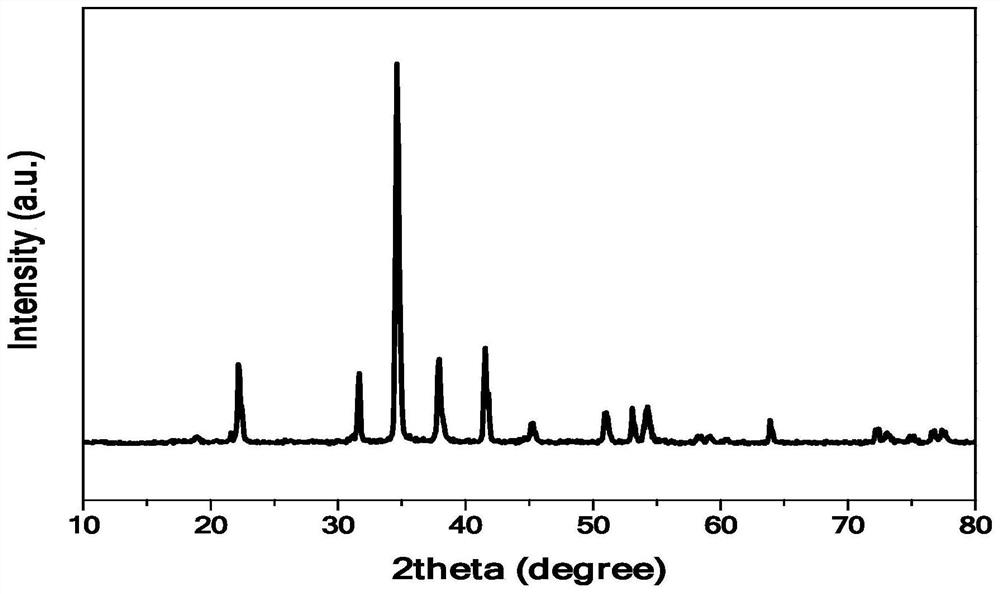
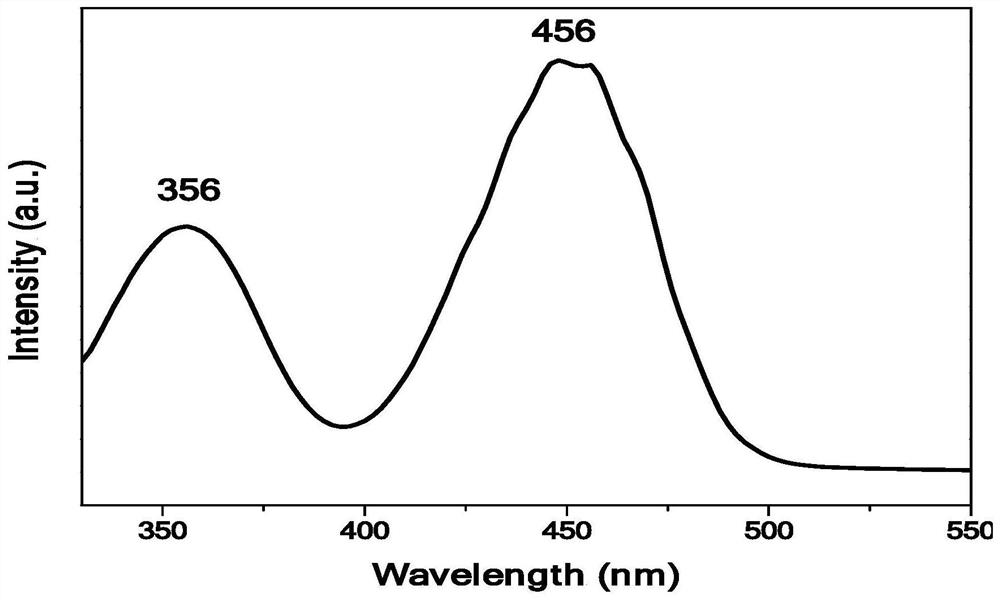
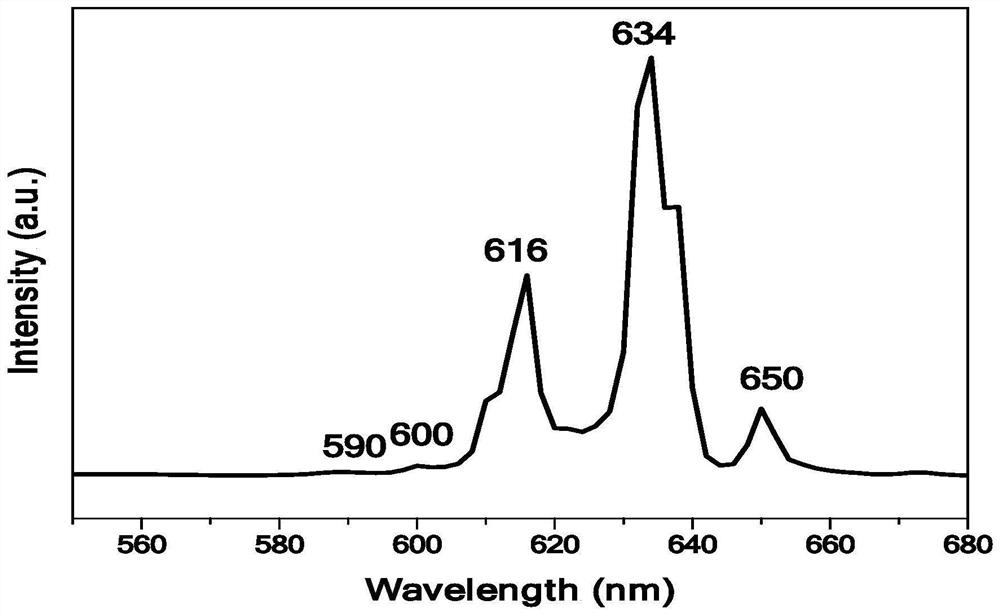
[0048] combined with Figure 1-5 As shown in the detection results, it can be seen that the red fluorescent powder KHF prepared by the preparation method of the present invention is 2 :Mn 4+ , the excitation peaks are located at 356nm and 456nm, and the emission spectrum is emitted...
Embodiment 2
[0051] A preparation method of fluoride-based fluorescent powder, carried out as follows:
[0052] (1) Preparation of Na 0.01 K 0.99 HF 2 :Mn 4+ : Add NaF to KF to form a mixture, the molar percentage of NaF is 1%, and the mixture is added to KMnO 4 It reacts directly with the mixed solution formed by 26% HF at normal temperature and pressure. After the reaction is completed, it is filtered, washed and dried. The mixture, HF and KMnO 4 The dosage relationship is 150:250:6;
[0053] (2) Mn 4+ Transfer: Na 0.01 K 0.99 HF 2 :Mn 4+ Dissolve in solvent distilled water, add fluoride matrix K 2 TiF 6 Place the reaction, wash, filter and dry after the reaction to obtain a new doped Mn 4+ The red phosphor product K2 TiF 6 :Mn 4+ , KHF 2 :Mn 4+ 、K 2 TiF 6 The molar ratio of matrix and distilled water is 1:4:80.
[0054] Na prepared by embodiment 2 0.01 K 0.99 HF 2 :Mn 4+ The KHF prepared by red fluorescent powder and embodiment 1 2 :Mn 4+ Compared with that, the...
Embodiment 3
[0056] A preparation method of fluoride-based fluorescent powder, carried out as follows:
[0057] (1) Preparation of KHF 2 :Mn 4+ : KF·2H 2 O joined KMnO 4 React directly with the mixed solution formed by HF with a mass concentration of 20% at normal temperature and pressure, filter, wash and dry after the reaction, KF, HF and KMnO 4 The molar ratio is 1500:2500:1;
[0058] (2) Mn 4+ Transfer: KHF 2 :Mn 4+ Dissolve in solvent distilled water, add fluoride matrix BaGeF 6 Place the reaction, wash, filter and dry after the reaction to obtain new doped Mn 4+ The red phosphor product BaGeF 6 :Mn 4+ , KHF 2 :Mn 4 + , BaGeF 6 The molar ratio relationship between matrix and distilled water: 1:4:300.
[0059] combined with Figure 6-10 As shown in the detection results, it can be seen that the red fluorescent powder KHF prepared by the preparation method of the present invention is 2 :Mn 4+ , the excitation peaks are located at 364nm and 462nm, and the emission spect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com