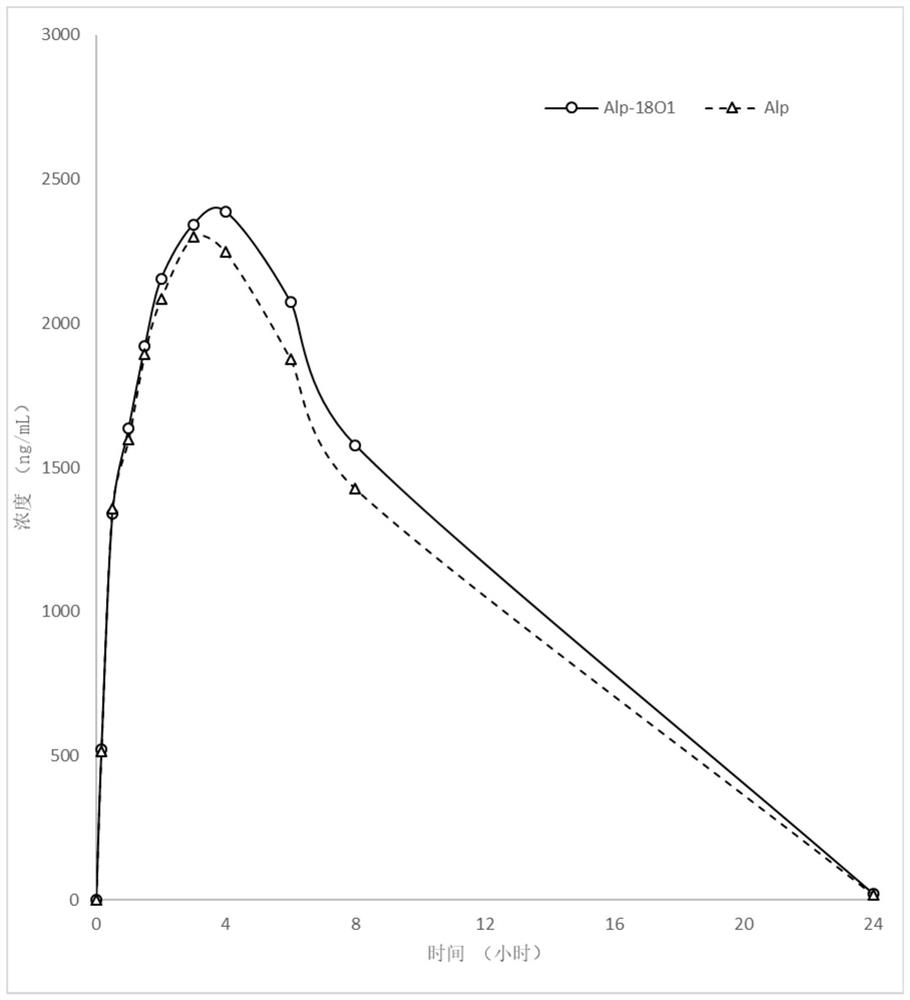
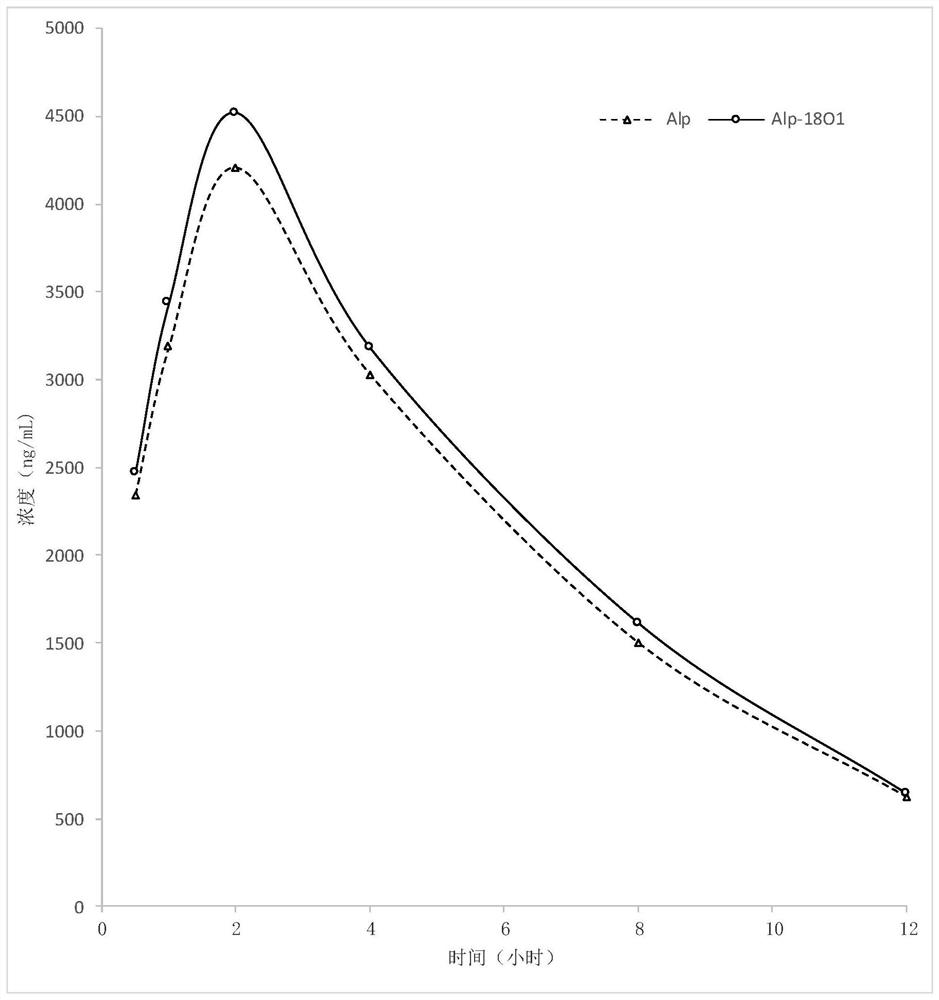
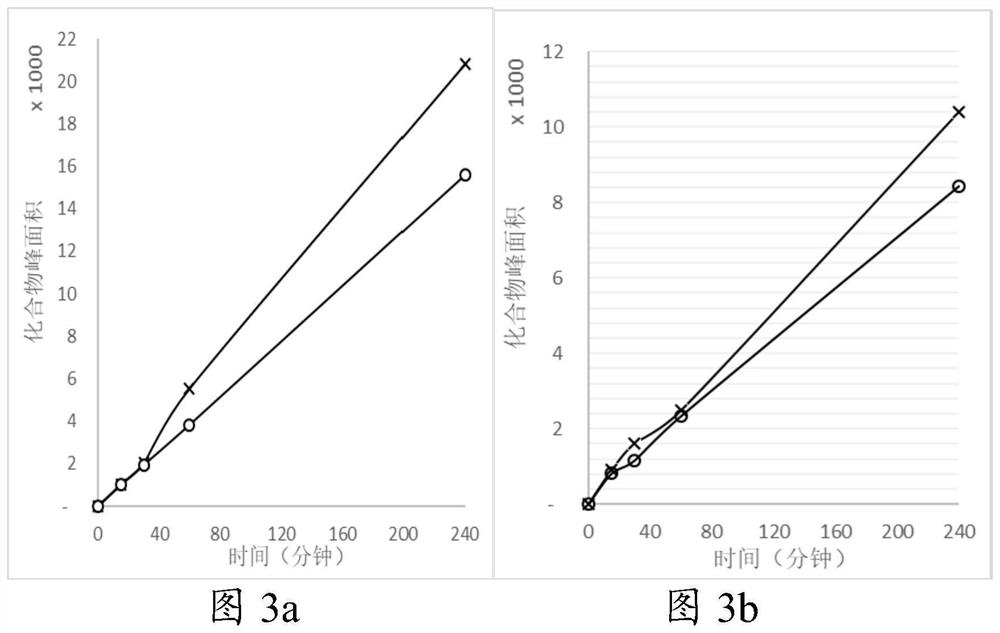
Compound containing amide functional group of stable heavy isotope and application thereof
A compound and functional group technology, applied to compounds containing stable heavy isotope amide functional groups and their application fields, can solve problems such as seldom use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Example 1: N-(4-methyl-5-(2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridine)-2-thiazole)aminocarbonyl- L-proline- 18 O-amide (Compound 1 or Alpelisib- 18 o 1 ) preparation
[0140] Under nitrogen protection, (S)-1-N-Boc-2-pyrrolidinecarbonitrile (100mg, 0.51mmol), palladium acetate (12mg, 0.051mmol), 2,2-bipyridine (8mg, 0.051mmol) added to H 2 18 In O (98% oxygen-18 abundance, 0.5 mL), the reaction was stirred at 60° C. for 24 hours in a sealed tube. The reaction solution was cooled to room temperature and transferred to a flask, concentrated under vacuum, and the residue was purified by column chromatography (mobile phase, dichloromethane:methanol=100 / 0~50 / 1), and finally N-Boc-L- Proline- 18O-amide (48 mg; oxygen-18 abundance, 97.3%).
[0141] N-Boc-L-proline- 18 O-amide (48mg, 0.22mmol) was dissolved in dichloromethane (0.5mL), then hydrochloric acid / dioxane solution (4M, 0.5mL) was added, stirred and reacted at 25°C for 1 hour, and then the reaction solutio...
Embodiment 2
[0146] Example 2: N-(4-methyl-5-(2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridine)-2-thiazole)aminocarbonyl- L-proline- 17 O-amide (Compound 2 or Alpelisib- 17 o 1 ) preparation
[0147] In addition to using O 17 - water (H 2 17 O) to replace the H used in Example 1 2 18 Compound 2 was obtained in the same manner as in Example 1 except for O.
Embodiment 3
[0148] Example 3: N-(4-methyl-5-(2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridine)-2-thiazole)aminocarbonyl- L-proline- 13 C 1 -amide (Compound 4 or Alpelisib- 13 C 1 ) preparation
[0149] In addition to using proline- 13 C 1 Compound 4 was obtained in a similar manner to Example 1 except that -amide hydrochloride was used as the starting material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com