Preparation method of novel polymerization inhibitor based on tetramethylpiperidine nitroxide free radical phosphite triester
A technology of tetramethylpiperidine nitroxide radical and phosphite triester, which is applied in the field of preparation of new polymerization inhibitors, can solve the problem of tetramethylpiperidine nitroxide radical phosphite triester polymerization inhibitor alkoxy In order to achieve the effects of high economic value, high yield and high current efficiency due to the lack of chemical research reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Put 110g of tetramethylpiperidine nitroxide free radical phosphite triester into the electrolytic reaction bottle, then respectively drop into 32g of water, 26g of ethanol, 22g of potassium phosphate, 10g of tetrabutylphosphorous hydroxide (40% aqueous solution), at 38 ℃ , 1200A / m 2 Electrolytic catalytic oxidation under current density, the anode material is DSA (shape stable anode), the cathode material is graphite plate, after 32 hours of electrolysis, the electrolyte is filtered to obtain the alkoxy group of tetramethylpiperidine nitroxide radical phosphite triester The product of the intermediate was 98g, the content (GC) was 96.82%, and the yield was about 88.32%.
[0030] .
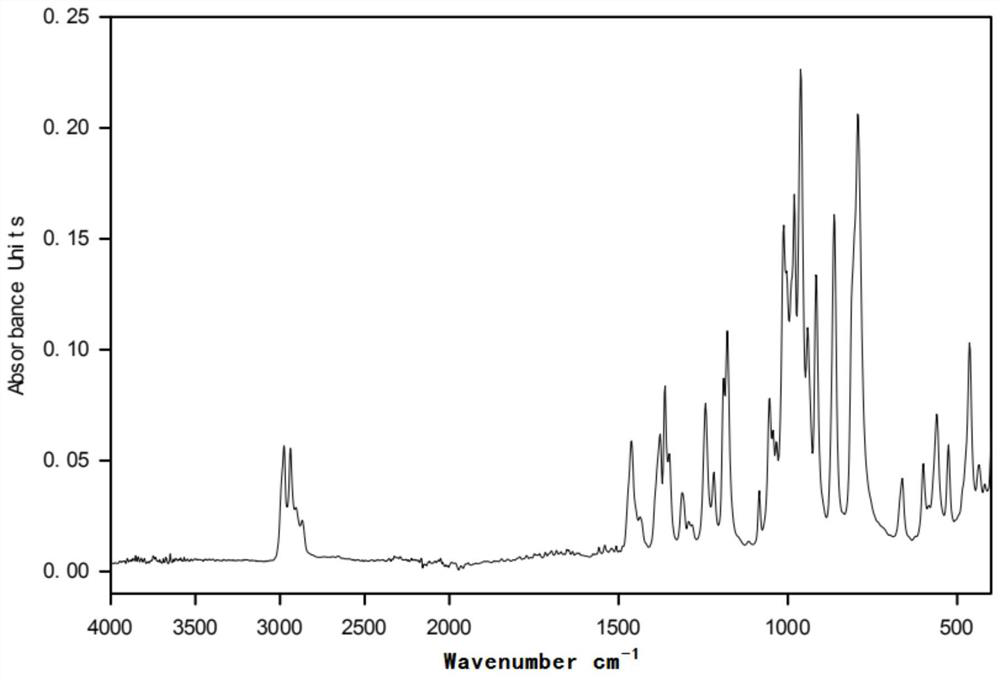
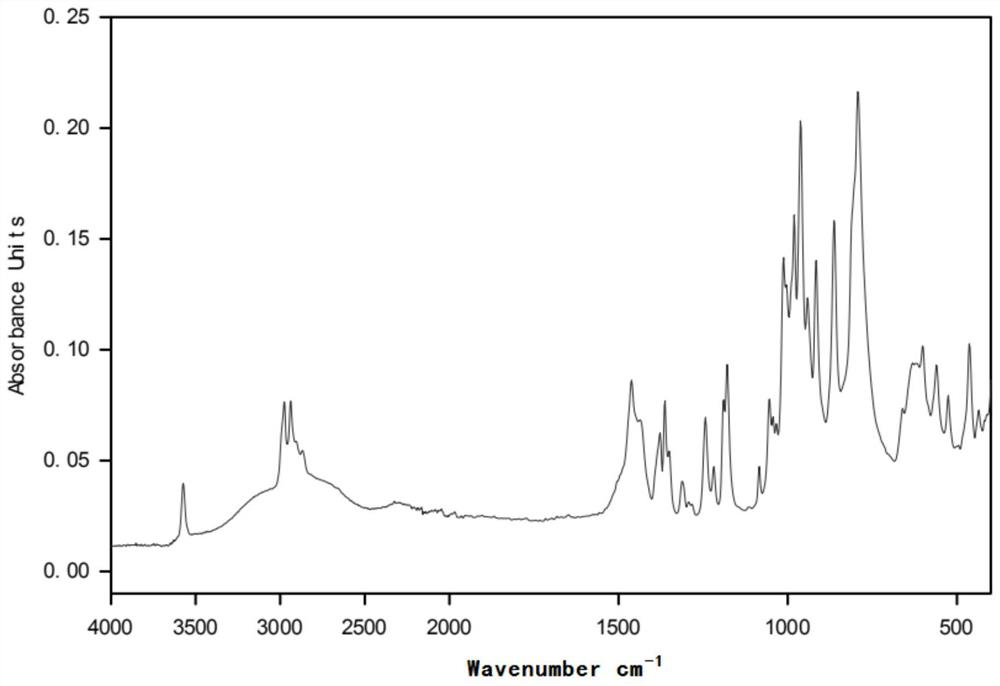
[0031] figure 2 for in (CH 3 CH 2 CH 2 CH 2 ) 4 The infrared spectrogram of the product prepared under the POH environment; before the electrolysis of the polymerization inhibitor 705, there was no hydroxyl (νOH) characteristic peak in the functional group region, and the alkoxy gr...
Embodiment 2
[0034] Put 110g of tetramethylpiperidine nitroxide free radical phosphite triester into the electrolytic reaction bottle, then drop into 30g of water, 22g of ethanol, 15g of potassium phosphate, 15g of tetrabutylphosphorus hydroxide (40% aqueous solution), at 38°C , 600A / m 3 Electrolytic catalytic oxidation under current density, the anode material is DSA (shape stable anode), the cathode material is graphite plate, after 32 hours of electrolysis, the electrolyte is filtered to obtain the alkoxy group of tetramethylpiperidine nitroxide radical phosphite triester The product 85g of the chemical intermediate, content (GC) 89.35%, yield is about 75%.
Embodiment 3
[0036] Put 110g of tetramethylpiperidine nitroxide free radical phosphite triester into the electrolytic reaction bottle, then drop into 40g of water, 30g of ethanol, 8g of potassium phosphate, 12g of tetrabutylphosphine hydroxide (40% aqueous solution), at 40°C , 600A / m 3 Electrolytic catalytic oxidation under current density, the anode material is DSA (shape stable anode), the cathode material is graphite plate, after 32 hours of electrolysis, the electrolyte is filtered to obtain the alkoxy group of tetramethylpiperidine nitroxide radical phosphite triester The product 80g of the chemical intermediate, content (GC) 91.33%, yield is about 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com