Process for producing sioc-bonded polyether siloxanes branched in the siloxane portion
A technology of siloxane and branched siloxane, which is applied in the direction of polymer adhesive additives, adhesive additives, improved hand feel fibers, etc., can solve problems such as unclear chemical properties, and achieve the effect of ensuring performance and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Preparation of Cyclic Branched Siloxane with Target D / T Ratio of 6:1
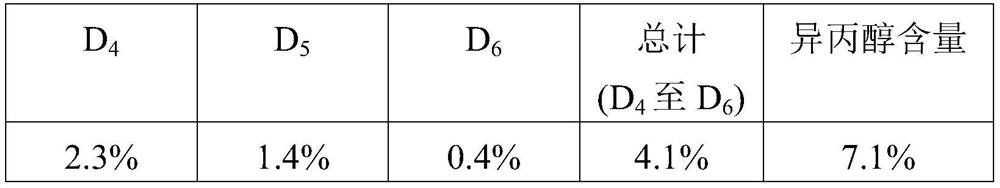
[0117] In a 10L four-necked round-bottomed flask equipped with a KPG stirrer and a reflux condenser, 783g (4.39mol) of methyltriethoxysilane and 978.7g (2.64mol) of decamethylcyclopentasiloxane were mixed under stirring Heat together to 60° C., admix with 2.98 g of trifluoromethanesulfonic acid, and equilibrate the mixture for 4 hours. Then 237 g of water and 59.3 g of ethanol were added and the batch was heated to reflux temperature for an additional 2 hours. Add 159.0g of water and 978.8g (2.64mol) of decamethylcyclopentasiloxane (D 5 ), and the reflux condenser was replaced with a distillation bridge, distilling out volatile components up to 90°C within the next hour. Then 3000 ml of toluene were added to the reaction batch, and the water still present in the system was removed by distillation in a water separator until the bottom temperature reached 100°C. The reaction mixture was cooled to ab...
Embodiment 2
[0119] Example 2 (Invention steps 1 and 2)
[0120] Preparation of acetoxy-terminated branched siloxanes
[0121] In a 500ml four-necked flask equipped with a KPG stirrer, an internal thermometer and a reflux cooler, first add 22.8g (0.223mol) of acetic anhydride and 101.2g of DT rings prepared in Example 1 under stirring (according to 29 The D / T ratio of Si-NMR spectrum=5.2:1, M=452.8g / mol and the ratio of SiOH / SiOEt part is 0.43mol%) and 125.9g decamethylcyclopentasiloxane (D 5 ), and mixed with 0.25 g (0.15 ml) of trifluoromethanesulfonic acid (0.1% by mass, based on the total batch), and then rapidly heated to 150° C. The initially slightly cloudy reaction mixture was kept at this temperature for 6 hours with continued stirring.
[0122] After cooling the batch, a colorless, clear, flowable liquid was isolated which 29 Si-NMR spectroscopy demonstrated the presence of Si-acetoxy groups in about 80% yield based on the acetic anhydride used, while a spectroscopically det...
Embodiment 3
[0123] Example 3 (Invention step 3)
[0124] Preparation of branched SiOC-bonded polyether siloxanes by post-neutralization in toluene
[0125] In a 500 ml four-necked flask with a KPG stirrer, internal thermometer and equipped with a reflux cooler, first 76.1 g of butanol with a molar mass of 1935 g / mol (molar mass determined by OH number) in 200 ml of toluene were charged under stirring The starting polyether alcohol (proportion of propylene oxide 100%), and mixed with 20 g of the trifluoromethanesulfonic acid-treated branched acetoxysiloxane prepared in Example 2. The reaction mixture was then heated to 40°C while stirring was continued for 1 hour. The reflux cooler was then replaced by a distillation bridge with a distillate receiver, and the batch was freed of toluene and acetic acid by distillation at 70°C with application of auxiliary vacuum.
[0126] After cooling, the distillation bottoms were mixed with 1.9 g of sodium bicarbonate and the salt was stirred for abo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com