Application of sennoside A in preparation of drugs for treating liver cancer
A technology for the treatment of liver cancer and sennoside, which is applied in the field of biomedicine and can solve problems such as poor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
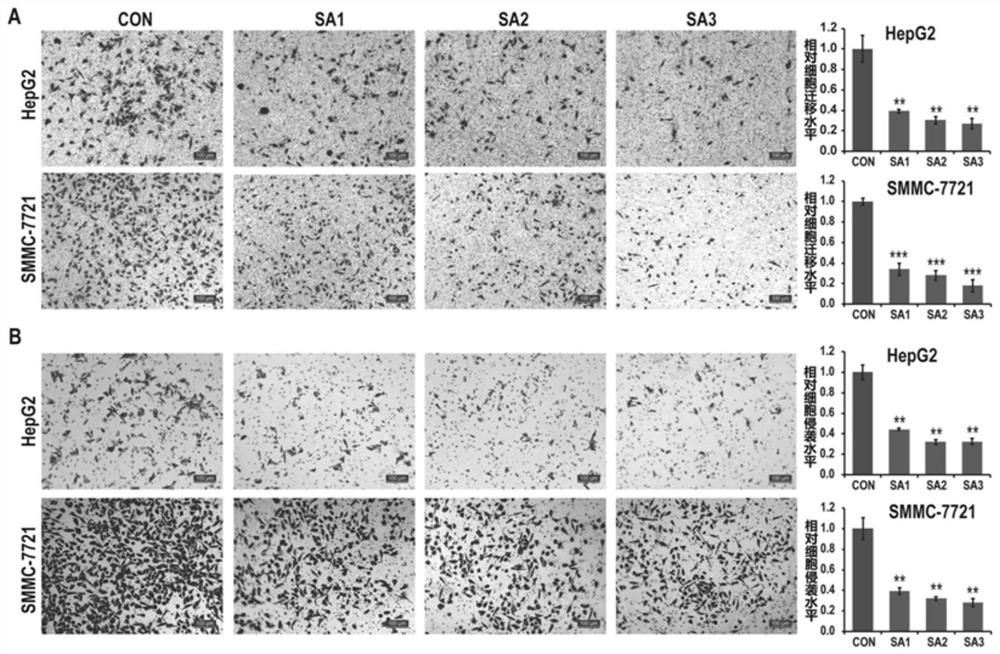
[0033] Example 1 The migration and invasion effects of sennoside A on two liver cancer cell lines were detected at the cell level.
[0034] 1. Experimental materials: human liver cancer cell line and its culture: HepG2 and SMMC-7721 cells were purchased from the cell bank of China Center for Type Culture Collection. Cells were cultured in DMEM-H (Gibco) medium containing 10% fetal bovine serum (Gibco) at 37°C, 5% CO 2 cultivated under conditions. Sennoside A was provided by Selleck Chemicals (Shanghai, China); unless otherwise noted, other reagents such as Trizol and DMSO were products of Sigma.
[0035]2. Experimental method: Transwell plates with 6.5mm transparent polycarbonate (PC) film and gel-coated Transwell plates with 6.5mm transparent polyester (PET) film were used respectively (article numbers 3422 and 354480 respectively; Corning-Costar , Cambridge, MA, USA) to detect cell migration and invasion abilities.
[0036] Specific steps are as follows:
[0037] (1) Pre...
Embodiment 2
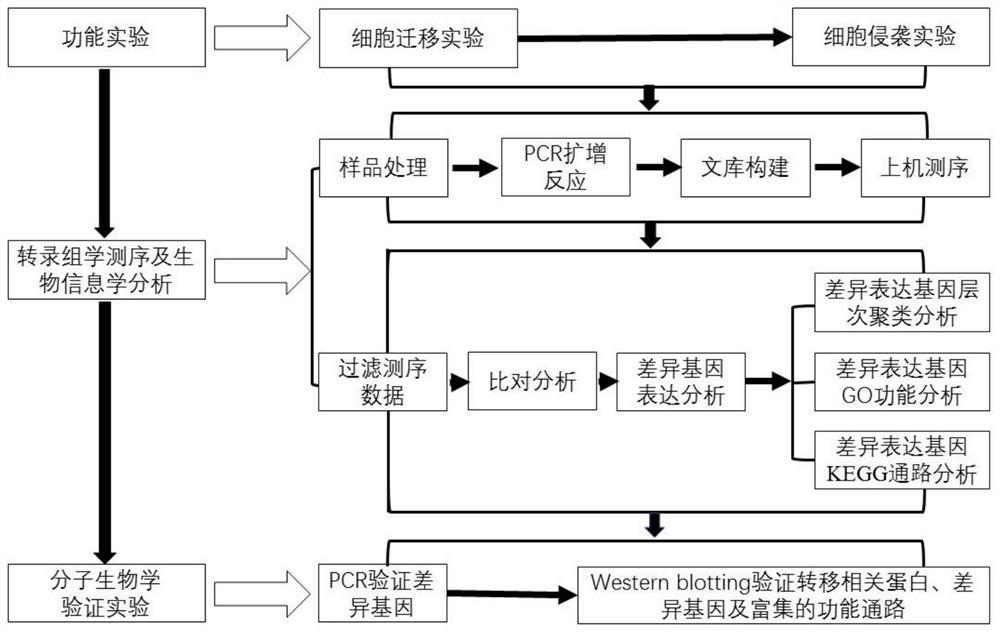
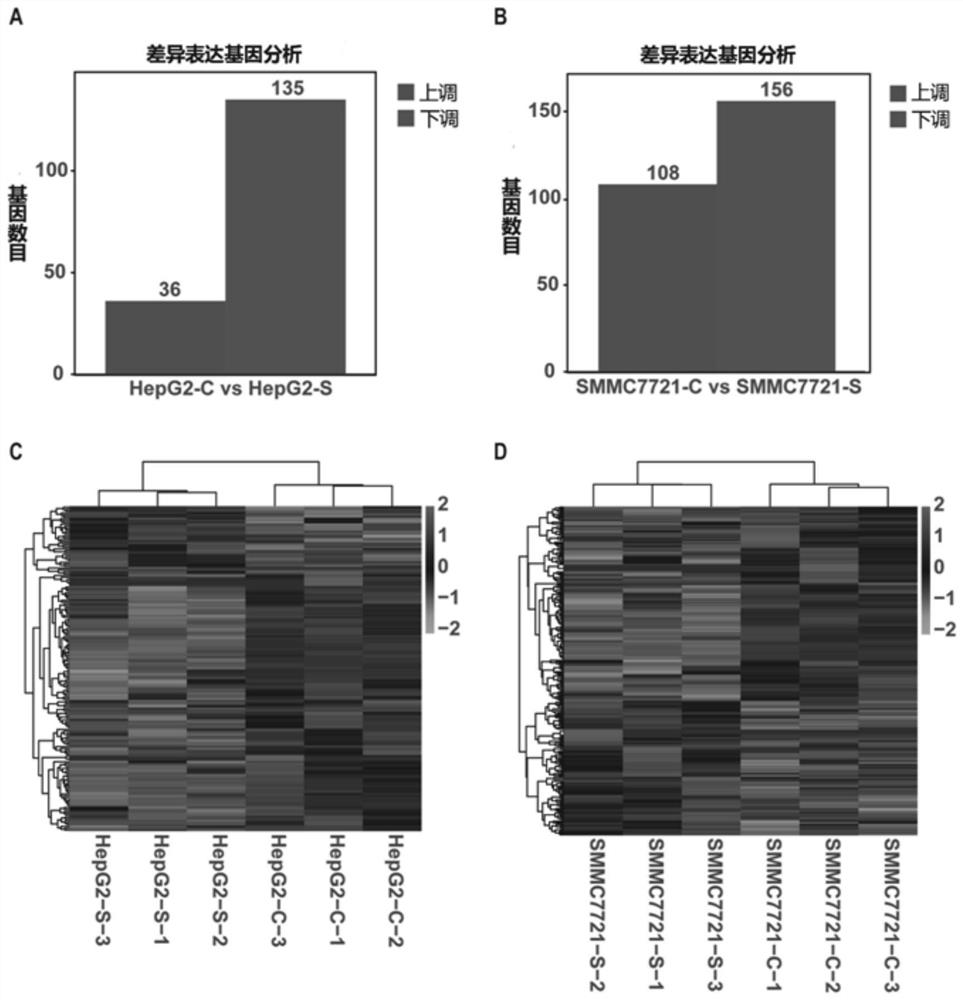
[0044] Example 2 Screening of sennoside A anti-metastasis targets of hepatocellular carcinoma by transcriptomics technology.
[0045] Step 1. Experimental process:
[0046] 1. mRNA isolation: Deactivate the total RNA sample, denature at a suitable temperature to open its secondary structure, and use oligo(dT) magnetic beads to enrich mRNA.
[0047] 2. mRNA interruption: Add an interruption reagent to the mRNA obtained in the previous step, react at an appropriate temperature for a certain period of time, and fragment the mRNA.
[0048] 3. cDNA synthesis: Add the pre-prepared one-strand synthesis reaction system to the interrupted mRNA, synthesize the first-strand cDNA according to the corresponding procedures on the PCR instrument, prepare the second-strand synthesis reaction system, react at an appropriate temperature for a certain period of time, and synthesize the second strand cDNA.
[0049] 4. End repair and adapter ligation: Prepare a reaction system, react at an appro...
Embodiment 3
[0073] Example 3 Molecular biological technology verification of the anti-metastasis target of sennoside A in hepatocellular carcinoma.
[0074] Step 1. Gene level: use RT-PCR technology to verify the shared differential genes related to tumor metastasis in two different liver cancer cell lines screened out by transcriptomics technology.
[0075] 1. Total RNA extraction
[0076] (1) Total RNA was extracted using RNA extraction kit (Promega, Shanghai, China) according to the product instructions.
[0077] (2) Detection of RNA concentration and purity. Use Nano-drop 2000 to detect RNA concentration and purity. When A260 / A280 is 1.8-2.0, it indicates that the purity of RNA is high. Such as A260 / A2802.2, indicating that RNA is degraded into single nucleotides. In both cases RNA should be re-extracted. The RNA concentration is preferably below 500 ng / μl.
[0078] 2. Reverse transcription
[0079] The reaction system of the kit (TOYOBO Bio-Technology, Shanghai, China) is as f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com