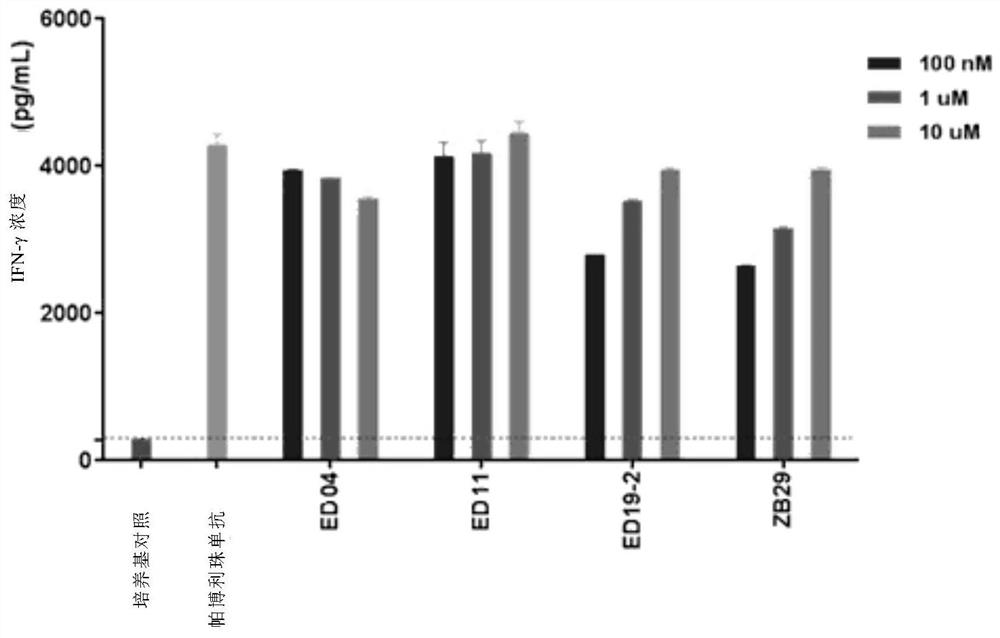
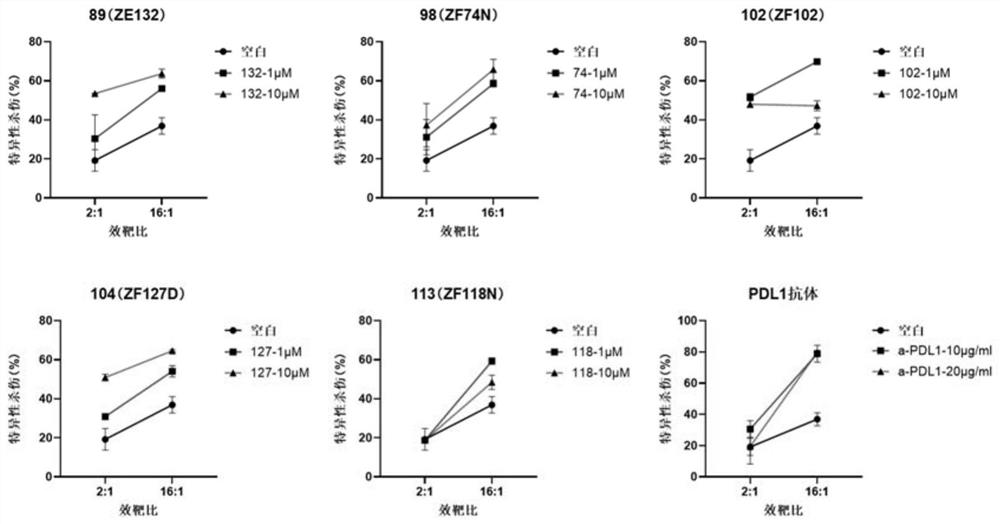
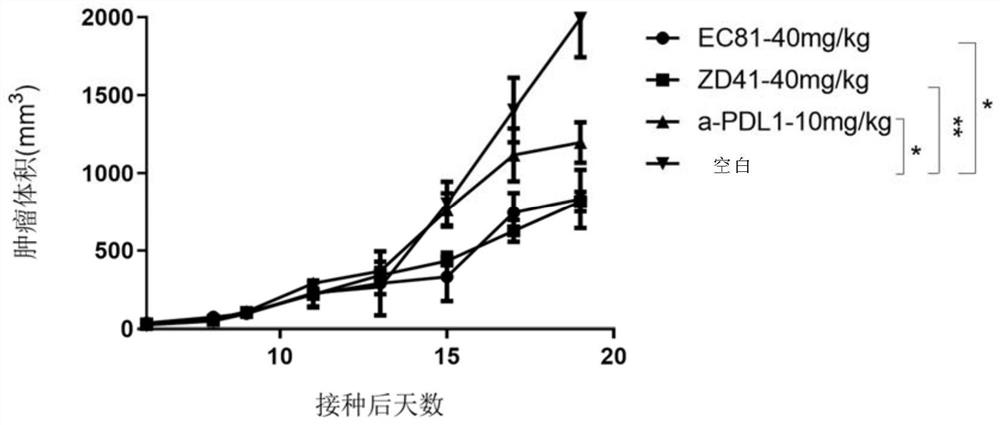
A compound with benzyloxy aromatic ring structure, its preparation method and use
A compound and alkyl technology, applied in the field of drug synthesis, can solve problems such as poor stability, easy immunogenicity, and high production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0241] The present invention also provides a preparation method of a pharmaceutical composition, comprising the steps of: mixing a pharmaceutically acceptable carrier with the compound of general formula (I) or its crystal form, pharmaceutically acceptable salt, hydrate or The solvates are mixed to form a pharmaceutical composition.
[0242] The present invention also provides a treatment method, which includes the step of: administering the compound of general formula (I) described in the present invention, its stereoisomer, enantiomer or pharmaceutically acceptable salt, or administer the pharmaceutical composition of the present invention for inhibiting PD1-PDL1 interaction.
[0243] The preparation of formula I compound
[0244] Methods of preparing compounds of Formula I are described in the following Schemes and Examples. Starting materials and intermediates were purchased from commercial sources, prepared by known procedures, or otherwise illustrated. In some cases, ...
Embodiment 1
[0380] Example 1: Synthesis of N-(3-((2-((3-cyanobenzyl)oxy)-4-((3-(2,3-dihydrobenzo[b][1,4] Dioxin-6-yl)-2-methylbenzyl)oxy)-5-methylbenzyl)(methyl)amino)propyl)but-2-yneamide (ZA89)
[0381]
[0382] Step 1: Synthesis of (3-bromo-2-methylphenyl)methanol (ZA01)
[0383] Dissolve 3-bromo-2-methylbenzoic acid (5.0g, 23.4mmol) in dry THF (tetrahydrofuran) (100ml), cool down in an ice-water bath, and then add LiAlH 4 (1.06g, 27.9mmol), react overnight at room temperature. After the reaction, the reaction solution was poured into saturated NaHCO 3 Quenched in , filtered, rinsed with dichloromethane (DCM), and the organic phase was dried with anhydrous sodium sulfate and then spin-dried to obtain the target product (2.0 g). 1 H NMR (400MHz, Chloroform-d) δ7.53(dd, J=1.2,8.0Hz,1H),7.36–7.29(m,1H),7.07(t,J=7.8Hz,1H),4.72(s, 2H), 2.43(s, 3H).
[0384] Step 2: Synthesis of (3-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-methylphenyl)methanol (ZA03)
[0385] Dissolve ZA01 (198mg, 1....
Embodiment 2
[0394] Example 2: Synthesis of 3-((5-((3-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-methylbenzyl )oxy)-4-methyl-2-((((2S,3R,4R,5R)-2,3,4,5,6-pentahydroxyhexyl)amino)methyl)phenoxy)methyl ) Benzonitrile (ED01)
[0395]
[0396] ZA80 (0.08mmol, 40mg), D-glucosamine (0.33mmol, 60mg) were dissolved in THF (4mL) and methanol (4mL), stirred at room temperature for 1 hour, and NaBH was added 4 (80mg, 2.1mmol), stirred overnight. After the reaction was completed, it was quenched with dilute hydrochloric acid, concentrated to obtain the crude product, and the crude product was purified by reverse liquid phase to obtain the trifluoroacetate (3.1 mg) of the target compound. 1 H NMR (400M, MeOD-d4): 7.90(s, 1H), 7.82(d, J=7.98Hz, 1H), 7.71(d, J=7.84Hz, 1H), 7.59(t, J=7.70Hz, 1H), 7.34(dd, J=7.07, 1.64Hz, 1H), 7.24-7.14(m, 3H), 6.88(d, J=8.03Hz, 1H), 6.80(s, 1H), 6.76-6.71(m ,2H),5.30(s,3H),5.15(s,3H),4.28(s,4H),4.21(q,J=12.34Hz,2H),4.11-4.05(m,1H),3.85(dd, J=4.44,1.36Hz,1H),3.77(dd,J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com