Recombinant pseudorabies live vaccine for preventing African swine fever, and preparation method of recombinant pseudorabies live vaccine
A technology for recombinant pseudorabies and African swine fever, applied in the fields of botanical equipment and methods, biochemical equipment and methods, vaccines, etc., can solve problems such as slow transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
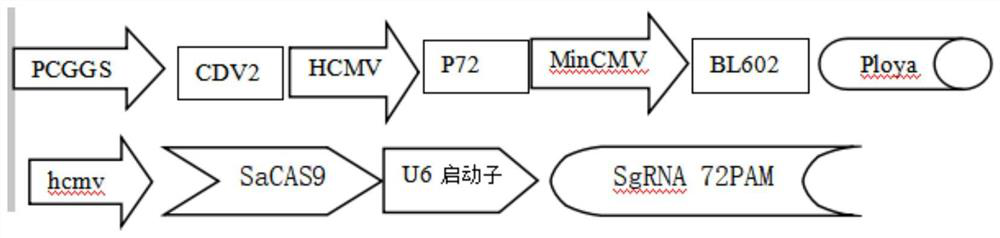
[0076] Example 1 - Obtaining the coding genes of recombinant CDV2 / P72 / BL602 and sacas9SgRNA. In order to increase the recombination rate, the CDV2 / P72 / BL602 and sacas9SgRNA expression cassettes were placed in the recombinant homology arms gl and US2 genes. The specific gene position in the recombination arm is shown in Table 2.
[0077] Table 2 Recombinant vector design
[0078] name 1 Gene iGO 1-1121 promoter 1 1122-1671 Cdv2 gene 1672-2754 promoter 2 2755-3044 P72 gene 3045-4730 promoter 3 4731-4279 BL601 4280-6872 WPRE terminator 6873-7463 promoter 4 7464-8077 Sacag gene 8079-11248 polya terminator 11249-11459 U6 promoter P72PAMgRNA 11460-11872 US2 homology arm 11873-12881
[0079] To clone the optimized antigen gene shown in SEQ ID No.1, the expression cassette containing CDV2 / P72 / BL602 and sacas9SgRNA is cloned into the recombinant sequence containing gl and US2, and its coding s...
Embodiment 2
[0080] Embodiment 2——recombinant virus construction
[0081] 1 Construction of recombinant CDV2 / P72 / BL602 and sacas9SgRNA gene recombinant vector
[0082] 1.1 Use PCR to amplify the gI gene US2 gene of the laboratory PRV virus, use the 3-terminal gI gene as the homology arm and the 5-terminal US2 gene as the homology arm gene, and introduce the Mlu1 / Ase1 restriction site at the same time, the gI gene and the US2 gene Introduce Ase1 and Mlu1 enzyme digestion sites in the middle, use gI and US2: to splice into recombinant arm genes, connect pEGFP vector, sequence and store correctly, shake the bacteria to extract vectors and recover them with Mlu1 and Ase1 enzyme digestion gels for recombinant CDV2 / P72 / BL602 expression box and sacas9SgRNAPAM gene expression box.
[0083] Primer sequence GIF: ggaggcgcgc cggct attaa t 21 (Sequence 5)
[0084] GIR: cgagccgggg gagatacgcg t 21 (sequence 6).
[0085] 1.2 Expression CDV2 / P72 / BL602 gene expression frame expansion (gene synthesi...
Embodiment 3
[0120] Embodiment 3——The effect of rPRV TIE1872V2Sag72 strain virus on pseudovirus interferon simulation determination
[0121] 1. Constructed with fluorescent P72
[0122] The wild-type P72 gene (SEQ ID NO: 19) was cloned into PDC315 to construct the MINIP72egfp gene vector to ensure no frame shift, and the adenovirus was packaged after correct sequencing.
[0123] sequence 19
[0124]
[0125] 2. BHK suspension cells were co-transfected with rPRV and MINIP72egfp-carrying adenovirus as the experimental group, and common PRV was used as the negative control to ensure that the sample volume of adenovirus in the two groups was the same, and the fluorescence values of diluted detection and undiluted were weaker than wild PRV Virus experimental group, the results are as follows Figure 6 ; It shows that the sacas9SgP72PAMRNA original of the recombinant virus group works normally and can cut the P72 gene. If the wild virus encounters this working original, it will interfere ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com