Utilization of cilastatin in inhibiting renal disorder
A technology for cilastatin and renal injury, which is applied in the application field of cilastatin in inhibiting renal injury to achieve the effect of avoiding renal injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0129] Hereinafter, although this invention is demonstrated based on an Example, this invention is not limited to these Examples.
[0130] Among the iodine-based contrast agents used, iomeprol is the Japanese Pharmacopoeia iomeprol injection (“Iomeron (registered trademark) 350” (Bracco-Bracco-Eisai Co., Ltd.)), and iohexol is the Japanese Pharmacopoeia iodine Hexylene injection (“Omnipaque (registered trademark) 350” (Daiichi Sankyo Co., Ltd.)), and iopamiron (registered trademark) 370” (Bayer Yakuhin, Ltd.) ), iodixanol is iodixanol injection "Visipaque (registered trademark) 320" (Daiichi Sankyo Co., Ltd.), ioversol is ioversol injection "Optiray (registered trademark) 350" (Fuji Pharmaceutical Industry Co., Ltd.), iopromide is iopromide injection ("Iopromide 370 injection "FRI"" (FUJIFILMRI Pharma Co., Ltd. / Input source: Bayer Yakuhin, Ltd.)). In addition, the cilastatin used was cilastatin sodium (Sigma-Aldrich Japan K.K.).
[0131] The animal species used was C57BL / 6J ...
reference example 1
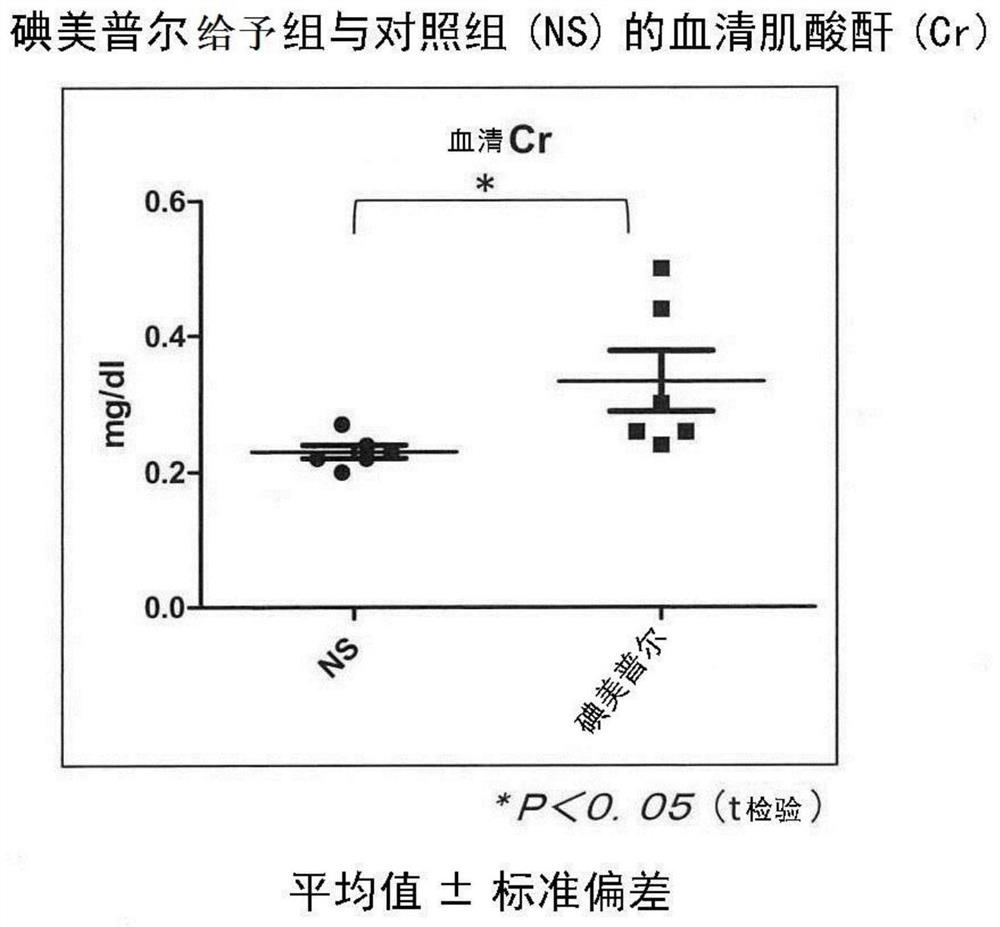
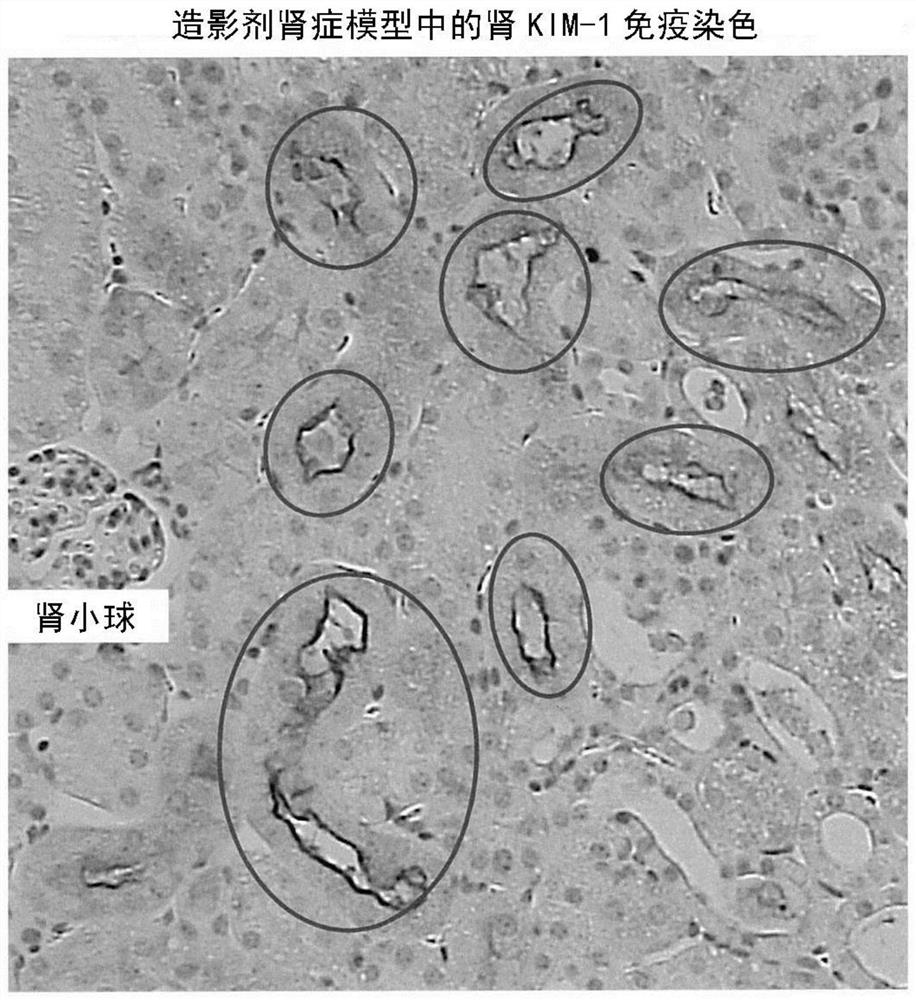
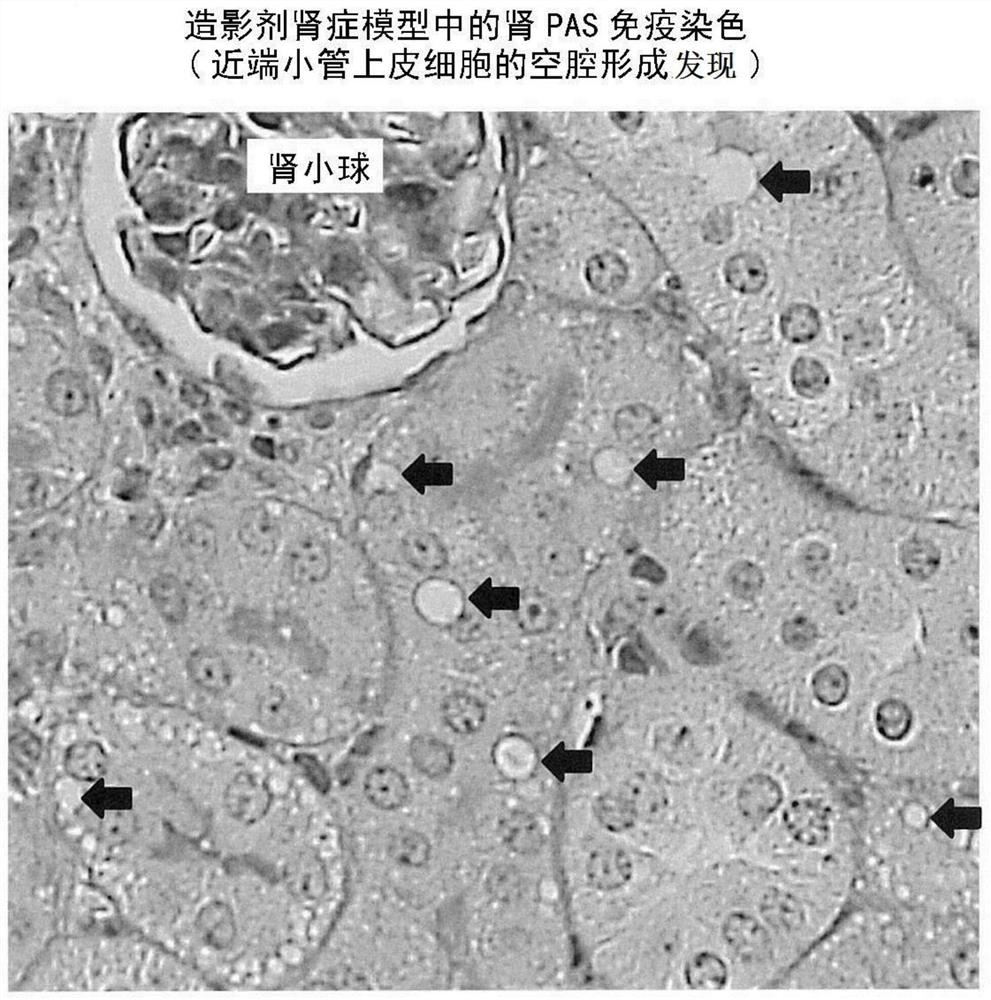
[0133] A mouse model of contrast-induced nephropathy was established.
[0134] method
[0135] The left kidneys of C57BL / 6J mice (male, 9-12 weeks old, Charles River Laboratories International) were removed, and the right renal stem was ligated for 30 minutes 14 days later. Then perform reperfusion. In the iomeprol administration group, 200 μL of iomeprol was intravenously administered 24 hours after the start of reperfusion. In the control group, 200 μL of physiological saline was administered from the tail vein instead of iomeprol. Animals from each group were dissected and evaluated 48 hours after administration.
[0136] Urine was stored in metabolic cages 24 hours before dissection, and the right kidney was removed after blood was collected from the inferior vena cava during dissection. The collected blood was centrifuged at room temperature at 800 g for 30 minutes to separate and recover serum. The resulting urine / serum was stored at -80°C until analysis.
[0137...
reference example 2
[0143] Using kidney-specific complete megalin knockout mouse NDRG1-Cre and its control mice (non-induced), it was verified that the contrast agent (iodine) in Uptake in proximal tubule epithelial cells depends on megalinity.
[0144] EPMA is one of electron microprobe (EMP) devices that analyze constituent elements based on the wavelength and intensity of characteristic X-rays unique to each element generated by irradiating an object with electron beams. Iodine contained in (Norby et al., Scanning Microsc, 1990, Vol. 4, pp. 651-666). It is known that the contrast agent is not metabolized in the living body, and the behavior of the contrast agent itself can be verified by analyzing the distribution of iodine.
[0145] The "thin film quantification method" can be used for the analysis of biological samples such as kidney tissue sections. In EPMA analysis, the following relationship holds true when electron beam irradiation and X-ray detection are performed under the same condi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com