Preparation method of 4-bromo-6-chloropyridine-2-carboxylic acid
A technology of chloropyridine and carboxylic acid, which is applied in the field of compound synthesis, can solve the problems of unpublished 4-bromo-6-chloropyridine-2-carboxylic acid synthesis method, etc., and achieve the effect of cheap raw materials, easy to obtain raw materials, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
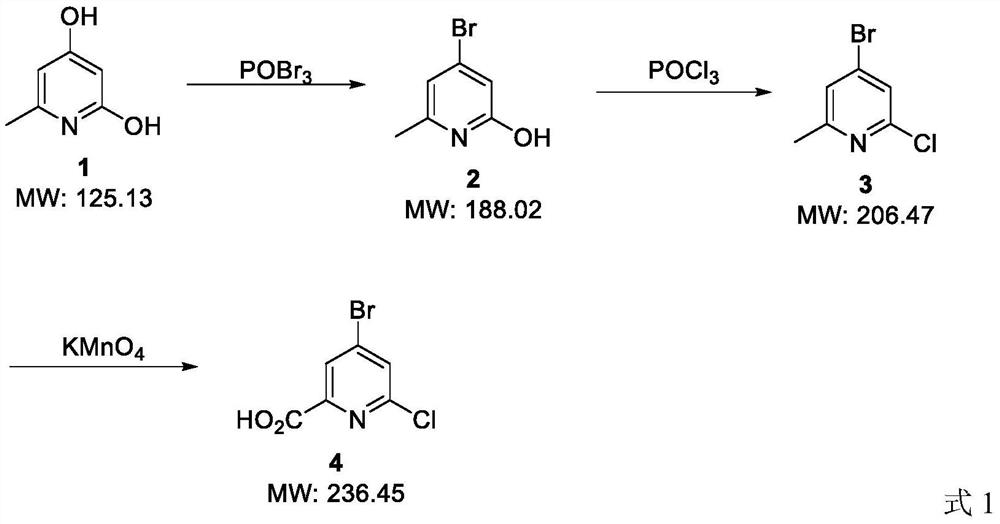
[0022] The preparation method of the 4-bromo-6-chloropyridine-2-carboxylic acid of the present embodiment comprises the following steps:
[0023] 1) Take a dry 100mL three-necked flask, add 12.5g of compound 1 and 50mL of DMF to it, compound 1 is 2,4-dihydroxy-6-picoline, put the three-necked flask in an ice-water bath, add 22.1g of phosphorus oxybromide, and then put the three-necked flask in an oil bath at 110°C, stir for 1 hour, and cool to 30°C. After cooling, add 60mL of water to the three-necked flask, then adjust the pH to 7 with sodium carbonate, precipitate a large amount of solid, filter, wash the filter cake once with 10mL of cold ethanol, wash once with 10mL of ether, and drain to obtain the compound 2 is 11.3g, the yield is 78%. Compound 2 is 2-hydroxy-4-bromo-6-methylpyridine.
[0024] 2) Take a dry 100mL three-necked bottle, add 5.64g of compound 2 and 30mL of DMF to it, place the three-necked bottle in an ice-water bath, add 7.7g of phosphorus oxychloride, and...
Embodiment 2
[0029] The preparation method of the 4-bromo-6-chloropyridine-2-carboxylic acid of the present embodiment comprises the following steps:
[0030] 1) Take a dry 100mL three-necked flask, add 12.5g of compound 1 and 50mL of DMF to it, compound 1 is 2,4-dihydroxy-6-picoline, put the three-necked flask in an ice-water bath, add 14.3g of phosphorus oxybromide, and then the three-necked flask was placed in an oil bath at 110°C, stirred for 1 hour, and cooled to 28°C. After cooling, add 60mL of water to the three-necked flask, then adjust the pH to 7 with sodium carbonate, precipitate a large amount of solid, filter, wash the filter cake once with 10mL of cold ethanol, wash once with 10mL of ether, and drain to obtain the compound 2 was 5.7 g, and the yield was 61%. Compound 2 was 2-hydroxy-4-bromo-6-methylpyridine.
[0031] 2) Take a dry 100mL three-necked bottle, add 5.64g of compound 2 and 30mL of DMF to it, place the three-necked bottle in an ice-water bath, add 15.4g of phospho...
Embodiment 3
[0034] The preparation method of the 4-bromo-6-chloropyridine-2-carboxylic acid of the present embodiment comprises the following steps:
[0035] 1) Take a dry 100mL three-necked flask, add 12.5g of compound 1 and 50mL of DMF to it, compound 1 is 2,4-dihydroxy-6-picoline, put the three-necked flask in an ice-water bath, add 22.1g of phosphorus oxybromide, and then put the three-necked flask in an oil bath at 110°C, stir for 1 hour, and cool to 30°C. After cooling, add 60mL of water to the three-necked flask, then adjust the pH to 7 with sodium carbonate, precipitate a large amount of solid, filter, wash the filter cake once with 10mL of cold ethanol, wash once with 10mL of ether, and drain to obtain the compound 2 is 11.3g, the yield is 78%. Compound 2 is 2-hydroxy-4-bromo-6-methylpyridine.
[0036] 2) Take a dry 100mL three-necked bottle, add 5.64g of compound 2 and 30mL of toluene to it, place the three-necked bottle in an ice-water bath, add 7.7g of phosphorus oxychloride,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com