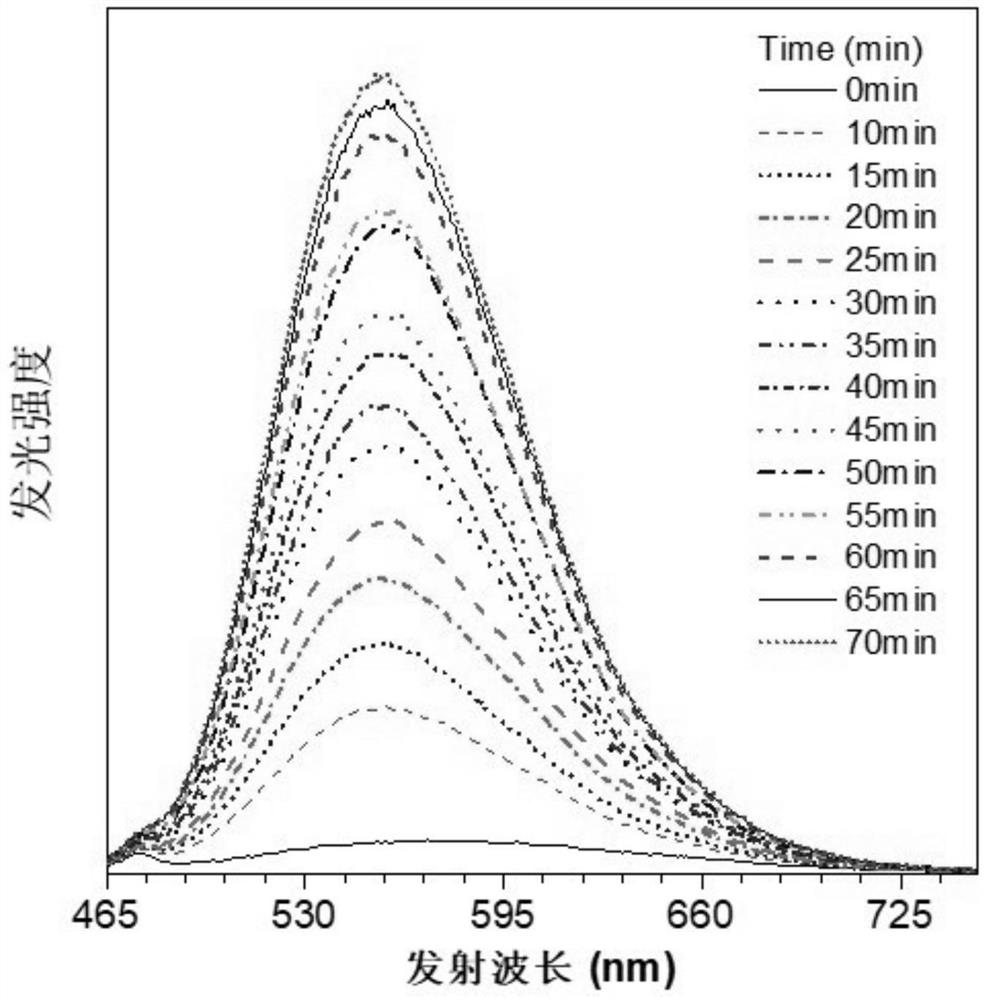
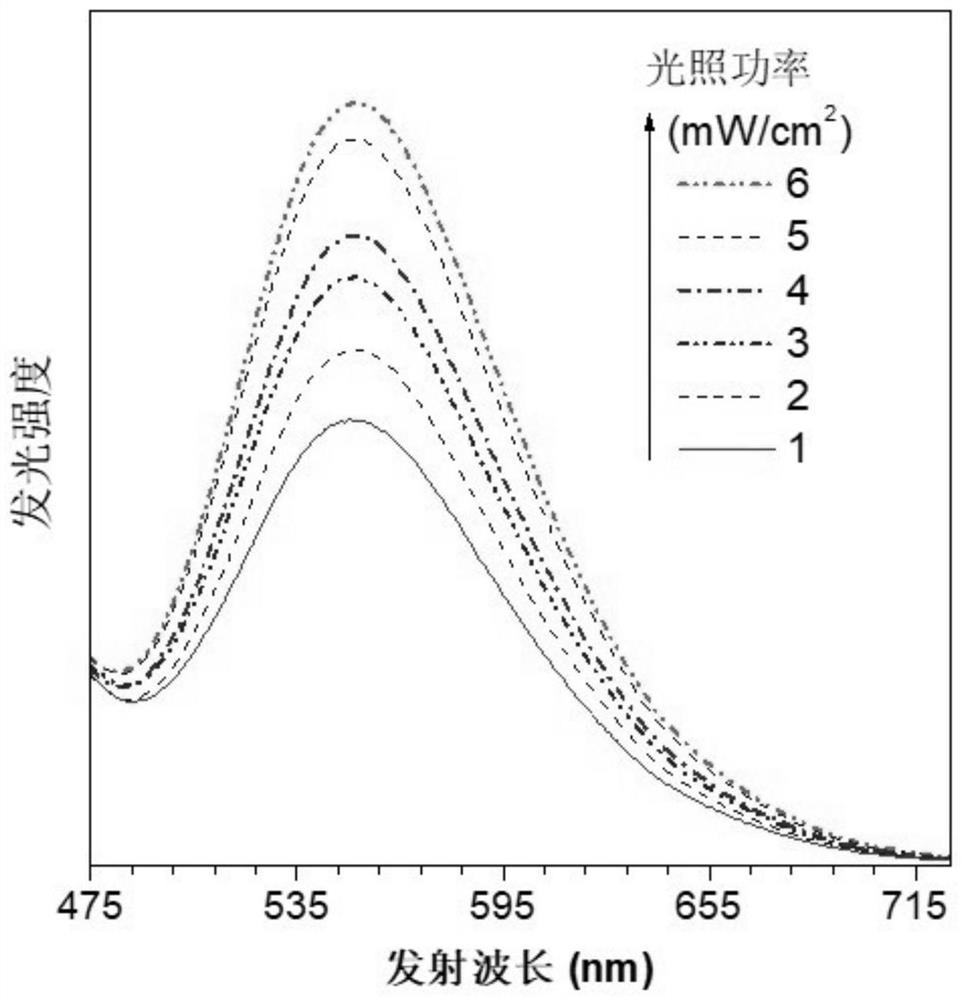
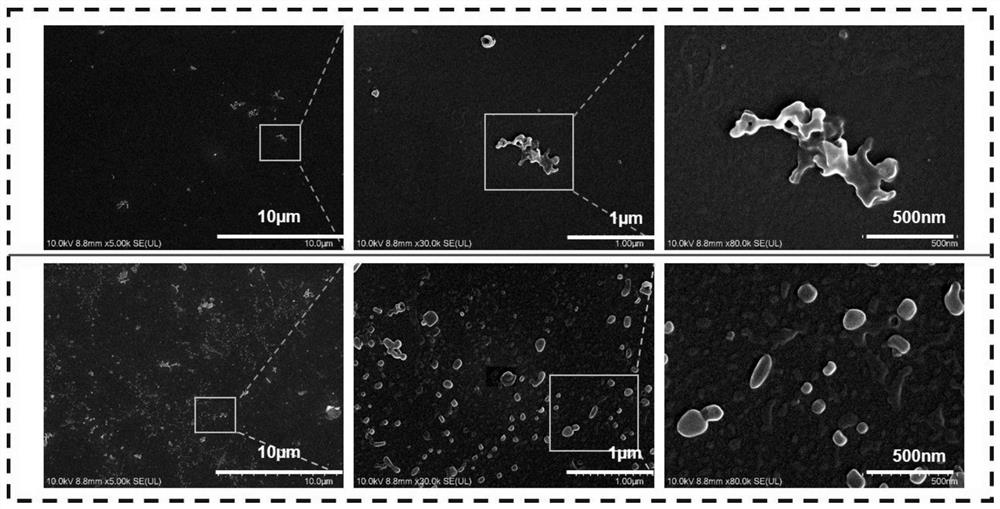
Photoinduced crystallization isoquinoline salt compound, preparation method and application thereof, and preparation method of nanocrystal
An isoquinoline salt and light-induced technology, applied in the direction of organic chemistry methods, chemical instruments and methods, selection of crystallization auxiliary conditions, etc., can solve the problems of time-consuming and complicated operation, and achieve easy control of size and good biocompatibility The effect of high-efficiency and high-efficiency active oxygen generation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of the isoquinolinate compound (TIOdB) of light-induced crystallization:
[0046]
[0047] The synthetic route is as follows:
[0048]
[0049] (1) 4,4'-dimethoxy-benzophenone (2.42g, 10mmol), carbon tetrabromide (6.63g, 20mmol) and triphenylphosphine (10.5g, 40mmol) mixture was evacuated and filled with N 2 Three times, and then add 500mL of anhydrous toluene to react at 140°C for 4 days. After cooling to room temperature, the mixture was filtered, washed with dichloromethane, and the filtrate was collected and washed with water. The organic layer was separated and washed with Mg 2 SO 4 Dry and remove solvent by rotary evaporator. The residue was purified by silica gel column chromatography using petroleum ether as eluent to afford product 2 as off-white solid in 45% yield.
[0050] (2) Put 1,1-bis(4-methoxyphenyl)-2,2-dibromoethylene (0.67g, 2mmol) into a two-necked flask (250 ml), 4-formylphenylboronic acid (0.31 g, 2.5mmol), Pd(PPh 3 ) ...
Embodiment 2
[0053] The preparation of the isoquinolinate compound (TIOB) of light-induced crystallization:
[0054]
[0055] The synthetic route is as follows:
[0056]
[0057] (1) 4,4'-dimethoxy-benzophenone (2.42g, 10mmol), carbon tetrabromide (6.63g, 20mmol) and triphenylphosphine (10.5g, 40mmol) mixture was evacuated and filled with N 2 Three times, and then add 500mL of anhydrous toluene to react at 140°C for 4 days. After cooling to room temperature, the mixture was filtered, washed with dichloromethane, and the filtrate was collected and washed with water. The organic layer was separated and washed with Mg 2 SO 4 Dry and remove solvent by rotary evaporator. The residue was purified by silica gel column chromatography using petroleum ether as eluent to afford product 2 as off-white solid in 45% yield.
[0058] (2) Put 1,1-bis(4-methoxyphenyl)-2,2-dibromoethylene (0.67g, 2mmol) into a two-necked flask (250 ml), 4-formylphenylboronic acid (0.31 g, 2.5mmol), Pd(PPh 3 ) 4...
Embodiment 3
[0061] Preparation of Isoquinolinate Compounds (TABO) Induced by Light Crystallization
[0062]
[0063] The synthetic route is as follows:
[0064]
[0065] (1) The mixture of benzophenone (10mmol), carbon tetrabromide (20mmol) and triphenylphosphine (40mmol) was evacuated and filled with N 2 Three times, and then add 500mL of anhydrous toluene to react at 140°C for 4 days. After cooling to room temperature, the mixture was filtered, washed with dichloromethane, and the filtrate was collected and washed with water. The organic layer was separated and washed with Mg 2 SO 4 Dry and remove solvent by rotary evaporator. The residue was purified by silica gel column chromatography using petroleum ether as eluent to afford product 2 as off-white solid in 75% yield.
[0066] (2) Put the product (2mmol) obtained in the previous step into a two-necked flask (250 milliliters), 4-formylphenylboronic acid (2.5mmol), Pd(PPh 3 ) 4 (0.1 mmol), K 2 CO 3(6 mmol) in toluene (60 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com