Glycosyltransferase mutant and application thereof
A technology of glycosyltransferases and mutants, applied in the biological field, can solve the problems of non-specificity of substrates and weak catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0118] The above-mentioned method for preparing rebaudioside M can be carried out intracellularly or extracellularly. As a preferred mode of the present invention, a method for intracellular production of rebaudioside M is provided: the mutant glycosyltransferase UGT76G1 corresponding to the 284th position of SEQ ID NO: 1 mutated to Ser and the aforementioned "to Enzymes that convert rebaudioside A to rebaudioside D", "Enzymes that convert stevioside to rebaudioside A", "enzymes that catalyze the aglycone steviol to stevioside or rebaudioside A" And / or the gene encoding "enzyme converting rebaudioside A or stevioside into rebaudioside D" is transformed into host cells, and the cells are cultured to produce rebaudioside M.
[0119] In the present invention, a series of mutants that weaken the catalytic activity of the glycosyltransferase UGT76G1 are also provided, and the mutation occurs at position 147, 155, 146 or 380 corresponding to the sequence of SEQ ID NO: 1, etc., for e...
Embodiment 1
[0126] Example 1, UGT76G1 protein expression, purification, crystallization and structural analysis
[0127] 1. Construction process of wild-type UGT76G1 expression vector pQZ11
[0128]Using the codon-optimized UGT76G1 gene cloning vector as a template, specific primer pairs (Table 1) were used to amplify the target gene. The PCR product was cloned into the BamHI / HindIII site of the vector pETDuet1, and the obtained expression vector pQZ11 was verified by sequencing.
[0129] Table 1. Primers used in the construction of wild-type UGT76G1 expression vector
[0130]
[0131] 2. Protein expression and purification
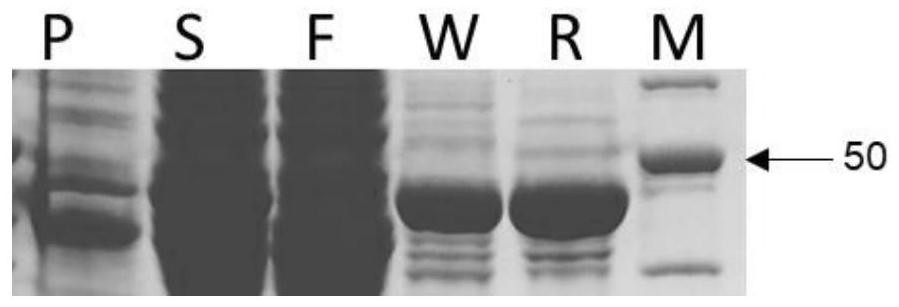
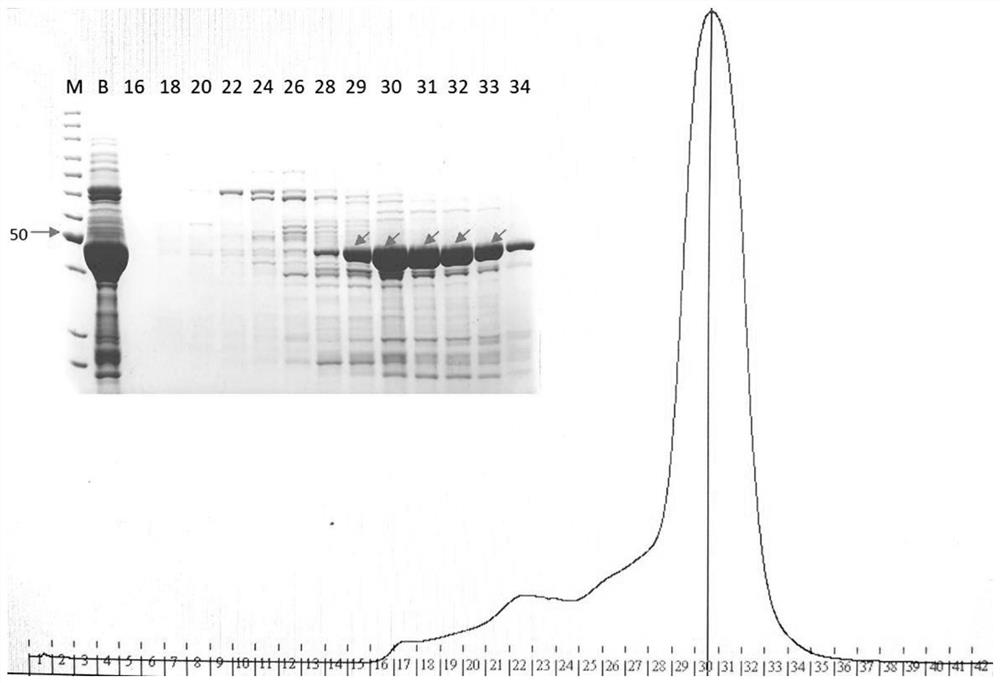
[0132] Transfer the overnight cultured E. coli BL21(DE3) carrying the wild-type UGT76G1 expression vector pQZ11 to 1L LB at 1% v / v, and cultivate to OD at 37°C and 200rpm 600 ≈1.0. Induced by IPTG with a final concentration of 0.1 mM, the cells were collected after culturing overnight at 16°C for 18 hours. Use resuspension buffer to resuspend cells, add 1mM P...
Embodiment 2
[0139] Embodiment 2, mutant protein construction and expression
[0140] According to UGT76G1-substrate rebaudioside B ( Figure 4 ) and UDP complex structure and repeated verification, the inventors are located in the substrate binding pocket, and have determined several key amino acids located in the substrate binding pocket ( Figure 5 ), which interact with the glycosyl donor, glycosyl acceptor or aglycon core, respectively. According to the function of amino acids involved in the glycosylation process, the inventors divided them into 4 categories (Table 2), carried out single-point or multi-point mutations on these amino acids, and determined the role of mutant proteins in participating in the glycosylation process through in vitro enzymatic tests. Changes in catalytic activity and substrate recognition specificity.
[0141] Table 2. Amino acid mutation sites
[0142]
[0143] 1. Mutant construction
[0144] Using primers containing point mutation sites (Table 3), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com