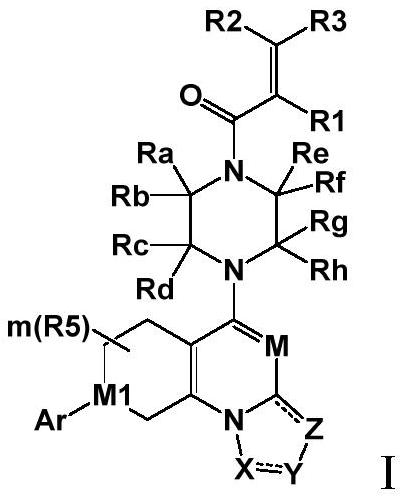
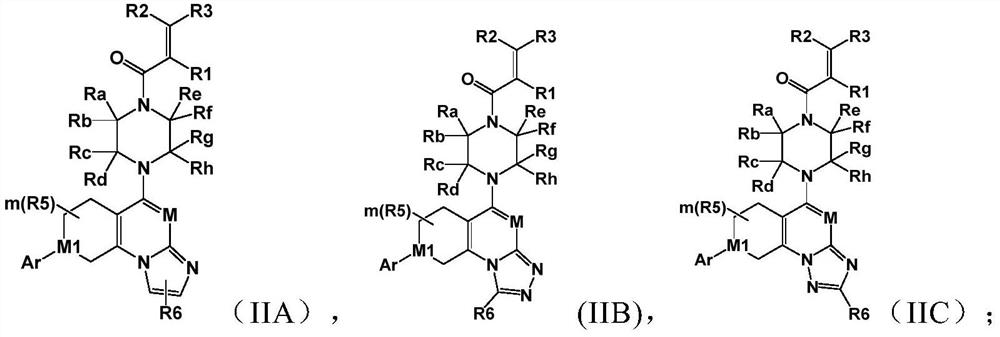
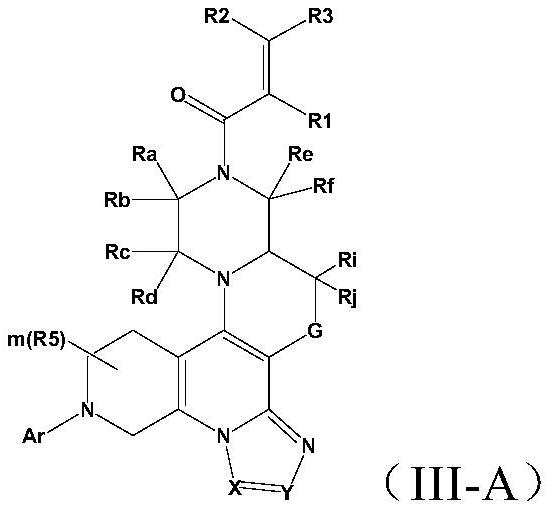
A class of saturated six-membered ring heterocyclic compounds, preparation method and use
A compound and six-membered ring technology, which is applied in the field of preparation, compounds that inhibit the activity of Ras mutant proteins, saturated six-membered rings and heterocyclic compounds, can solve the problems of unsuccessful development of targeted drugs and little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0202] Example 1: (S) -1- (4- (8- (5- methyl -1H- indazol-4-yl) -1- (1-methyl-pyrrolidin-2-yl) - 6,7 , 8,9-tetrahydro-pyrido [4,3-e] [1,2,4] triazolo [4,3-a] pyrimidin-5-yl) piperazin-1-yl) propan-2- - en-1-one
[0203]
[0204] Step: 2,4-dichloro-5,8-dihydro-pyrido [3,4-d] pyrimidin -7 (6H) - carboxylate (10.4g, 34.3 mmol) and piperazine - 1- carboxylic acid benzyl ester (7.5g, 34.1mmol) was dissolved in 1,4-dioxane (200mL) was added DIPEA (13.2g, 102mmol). At room temperature for 2 days, concentrated under reduced pressure, the residue was purified by column chromatography to give 4- (4 - ((benzyloxy) carbonyl) piperazin-1-yl) -2-chloro-5,8-dihydro-pyrido [ 3,4-d] pyrimidin -7 (6H) - carboxylate (11g, white solid). LC-MS: ESI [M + H] + = 488.5; 1 HNMR (400MHz, DMSO-D 6 ): Δ7.33-7.39 (m, 5H), 5.11 (s, 2H), 4.37 (brs, 2H), 3.50 (brs, 10H), 2.63-2.65 (m, 2H), 1.44 (s, 9H).
[0205] Step: 4- (4 - ((benzyloxy) carbonyl) piperazin-1-yl) -2-chloro-5,8-dihydro-pyrido [3,4-d] pyrimidin...
Embodiment 2
[0214] Example 2: Preparation of 1- (4- (8- (5-methyl -1H- indazol-4-yl) -6,7,8,9-tetrahydro-pyrido [4,3-e] [1, 2,4] triazolo [4,3-a] pyrimidin-5-yl) piperazin-1-yl) prop-2-en-1-one
[0215]
[0216] Step: 4- (2-hydrazino-7- (5-methyl-1 - ((2- (trimethylsilyl) ethoxy) methyl) lH-indazol-4-yl ) - 5,6,7,8-tetrahydro-pyrido [3,4-d] pyrimidin-4-yl) piperazine-1-carboxylic acid benzyl ester (0.5g, 0.79mmol) was dissolved in dichloromethane (15mL ) was added trimethyl orthoformate (340mg, 3.2mmol), stirred ten minutes, then trifluoroacetic acid (95mg, 0.83mmol). After continued stirring at room temperature for 1 hour and concentrated under reduced pressure, and the residue to give 4- (8- (5-methyl-1 isolated and purified by silica gel column chromatography - ((2- (trimethylsilyl) ethoxy) methyl yl) lH-indazol-4-yl) - 6,7,8,9-tetrahydro-pyrido [4,3-e] [1,2,4] triazole [4,3-a] pyrimidine 5-yl) piperazine-1-carboxylic acid benzyl ester (320mg, yellow solid). LC-MS: ESI [M + H] + = 654.7;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com