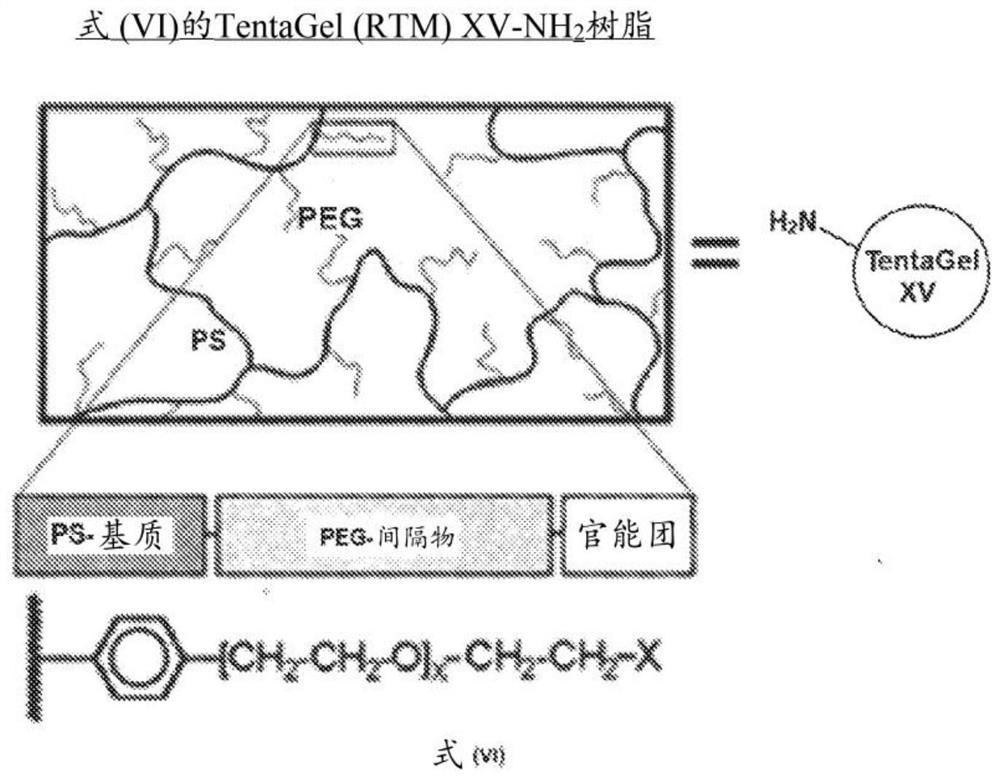
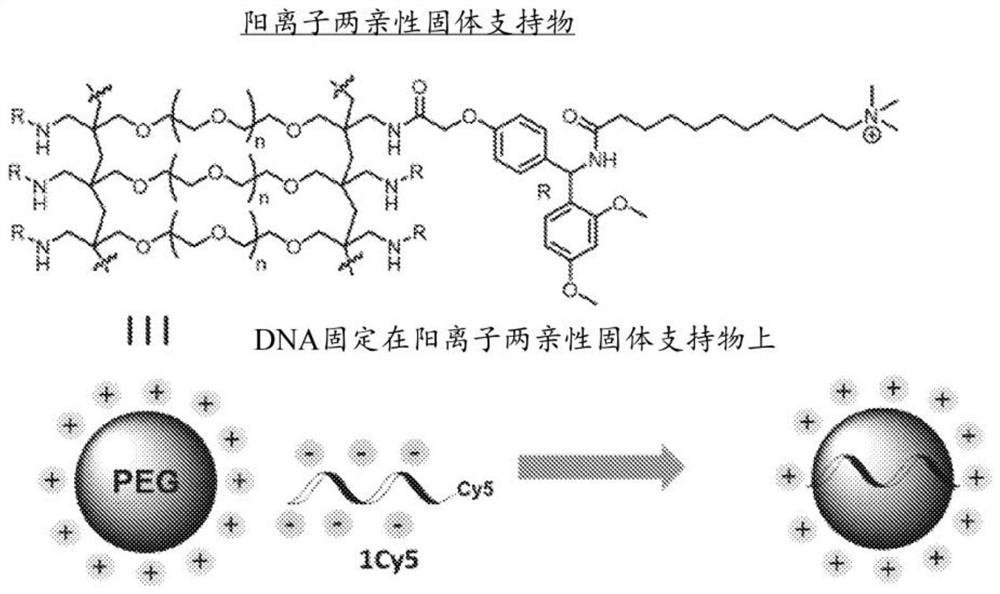
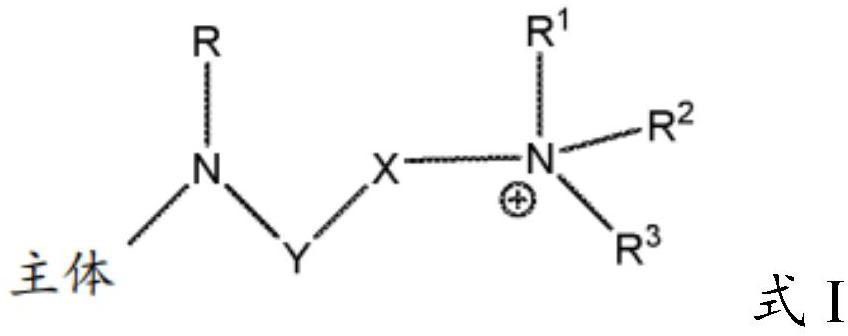
Solid support
A solid support, support technology, used in biochemical equipment and methods, microbial determination/inspection, instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0293] Example preparation
[0294] The following examples have been prepared, isolated and characterized using the methods disclosed herein.
[0295] abbreviation
[0296] Abbreviations as used herein are defined as follows: "1x" means once, "2x" means twice, "3x" means three times, "°C" means degrees Celsius, "aq" means aqueous, "Col" means column, "eq" means equivalent (equivalent or equivalents), "g" means gram (gram or grams), "mg" means milligram (milligram or milligrams), "nm" means nanometer (nanometer or nanometers), "L" means liter (liter or liters), "mL" or "ml" means milliliter or milliliters, "ul", "uL", "μl", or "μL" means microliter (microliter or microliters), "nL" or "nl" means nanoliter (nanoliter or nanoliters), "N" means normal, "uM" or "μM" means micromole, "nM" means nanomole, "mol" means mole (mole or moles), "mmol" means millimole (millimole or millimoles), "min" means minutes (minute or minutes), "h" or "hrs" means hours (hour or hours), "RT" means ...
example 1
[0350] Synthesis of Cationic Acid 10-Carboxyl-N,N,N-Trimethyldecane 1-Ammonium Bromide
[0351]
[0352] 11-Bromoundecanoic acid (Fluka CAS 2834-05-1) (8 g, 30.2 mmol) was dissolved in trimethylamine (35% in EtOH) (60 ml, 224 mmol), and the reaction vessel was sealed with a septum, at room temperature The solution was stirred for 48 hours. A white precipitate formed at this stage. The white precipitate was filtered and washed with cold EtOH. The collected white solid was recrystallized from EtOH, filtered and dried under reduced pressure to give pure 10-carboxy-N,N,N-trimethyldecane-1-ammonium bromide as a white powder.
[0353] 1 H NMR (400MHz, DMSO-d6) δ3.30-3.24 (m, 2H), 3.04 (s, 9H), 2.19 (t, J = 7.3Hz, 2H), 1.71-1.60 (m, 2H), 1.48 ( t, J=7.1 Hz, 2H), 1.27 (d, J=11.5 Hz, 12H).
[0354] UPLC-MS: 0.51 min; 244.3 (M)+. (2_MIN_REACTION_MONITORING_IPA): Waters UPLC Acquity; Column: Acquity UPLC BEH C18, 1.7μm, 2.1x50mm, at 80°C, Eluent A: H 2 O+0.05% HCOOH+3.75 mM amm...
example 2
[0356] Commercially available NovaPEG resin Rink amide was obtained. The resin NovaPEG resin Rink amide (250 mg) (0.250 g, 0.115 mmol) was swelled in DMF for 3 hours.
[0357] Convert the resin by adding the cationic acid:
[0358] (0.460 mmol), HOAt (0.063 g, 0.460 mmol), and DIC (0.072 mL, 0.460 mmol) were mixed in DMF (volume: 5 mL) / DMSO (volume: 3 mL). The resulting solution was added to the resin and allowed to react for 2 hours. This process was repeated once using only DMSO as solvent. The Kaiser test for free amines was negative.
[0359] Resulting Derivatized Resin:
[0360]
[0361] Wash four times with DMSO and four times with water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com