Medicine and food for preventing or treating COVID-19 and application thereof
A COVID-19 and drug technology, applied in the field of medicine, can solve the problems of toxicity, poor effect of viral pneumonia, and no obvious activity of COVID-19
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1: Application of lysozyme in patients with new coronary pneumonia and high-risk infection groups
[0088] During the COVID-19 epidemic, our company donated more than 2 million yuan of medicines to Hubei Province twice, including antibacterial drugs such as lysozyme buccal tablets and lysozyme enteric-coated tablets. We found that these drugs played a positive role in the fight against the new crown epidemic.
[0089] There were 9 newly diagnosed patients with new coronary pneumonia who took the lysozyme buccal tablets and / or lysozyme enteric-coated tablets provided by us. The dosage ranged from 0.5g to 2g per day. severe, and the other 8 were all mild and recovered later. The calculated severe conversion rate was 11.1%.
[0090] There are 3 people who lived and traveled in high-risk areas during the epidemic. They insisted on taking lysozyme buccal tablets and / or lysozyme enteric-coated tablets every day. Coronavirus, 0% infection rate.
[0091] According t...
Embodiment 2
[0092] Example 2: In vitro test of lysozyme against new coronavirus
[0093] The anti-COVID-19 novel coronavirus effect of lysozyme was studied with African green monkey kidney cells.
[0094] Drugs: lysozyme, monoammonium glycyrrhizinate.
[0095] Cells: Vero cells (VeroE6 cells).
[0096] Virus: COVID-19, a titer of 100TCID was used 50 .
[0097] Culture: sterile 96-well culture plate, add 100 μL to each well with a concentration of 2×10 5Cells / mL VeroE6 cells were cultured at 37°C for 24 hours. The culture plates were divided into blank control group, virus control group, low-dose lysozyme group, middle-dose lysozyme group, high-dose lysozyme group, low-dose monoammonium glycyrrhizinate group, and high-dose monoammonium glycyrrhizinate group. 3 holes. In addition to the blank control group, 100TCID was added to each group 50 Virus solution 100 μL / well, 5% CO at 37°C 2 After 2 hours of adsorption in the incubator, discard the cell culture medium in the culture plate....
Embodiment 3
[0103] Example 3: In vitro test of combination containing lysozyme against new coronavirus and inflammation caused by new coronavirus
[0104] To study the inhibitory effect of the combination containing lysozyme on the cytopathy caused by the COVID-19 virus, and the inhibitory effect on the inflammation caused by the COVID-19 virus.
[0105] Medicine, cell, virus etc. are all identical with embodiment 2.
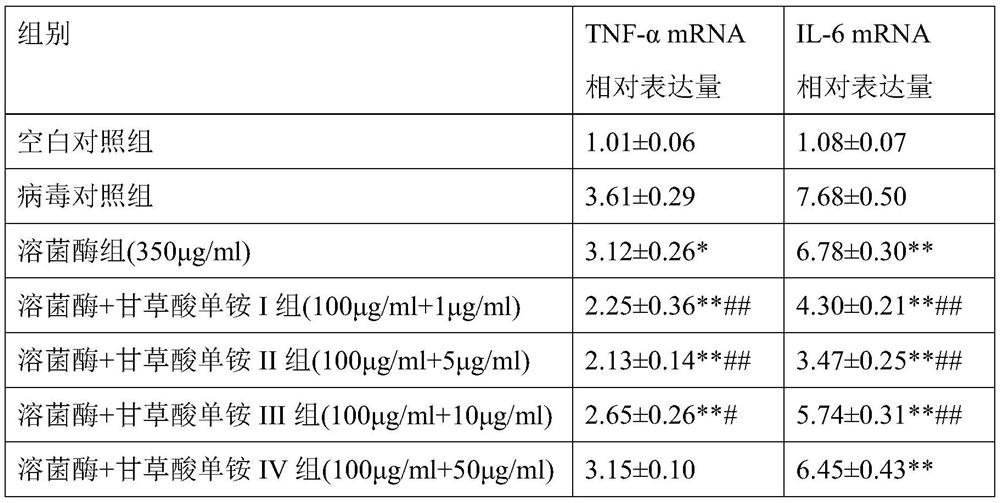
[0106] The grouping and dosage of the culture plate are: blank control group, virus control group, lysozyme group (350 μg / ml), lysozyme monoammonium glycyrrhizinate I group (100 μg / ml+1 μg / ml), lysozyme monoammonium glycyrrhizinate Group II (100 μg / ml+5 μg / ml), group III of lysozyme monoammonium glycyrrhizinate (100 μg / ml+10 μg / ml), group IV of lysozyme monoammonium glycyrrhizinate (100 μg / ml+50 μg / ml).
[0107] The method for observing cytopathic changes is the same as in Example 2, and the test results are shown in Table 2. In addition, non-pathological cells were colle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com