Novel method for preparing hydroxypyrimidine by continuously oxidizing dihydropyrimidinone
A technology for oxidizing dihydropyrimidinone and hydroxypyrimidine, which is applied in chemical instruments and methods, organic chemistry, chemical/physical processes, etc., can solve the problems of being unfriendly and not substantially improving the environment of excessive nitric acid, so as to reduce safety risks, The effect of reducing EHS safety risks and improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: technical scheme described in the present invention
[0056]
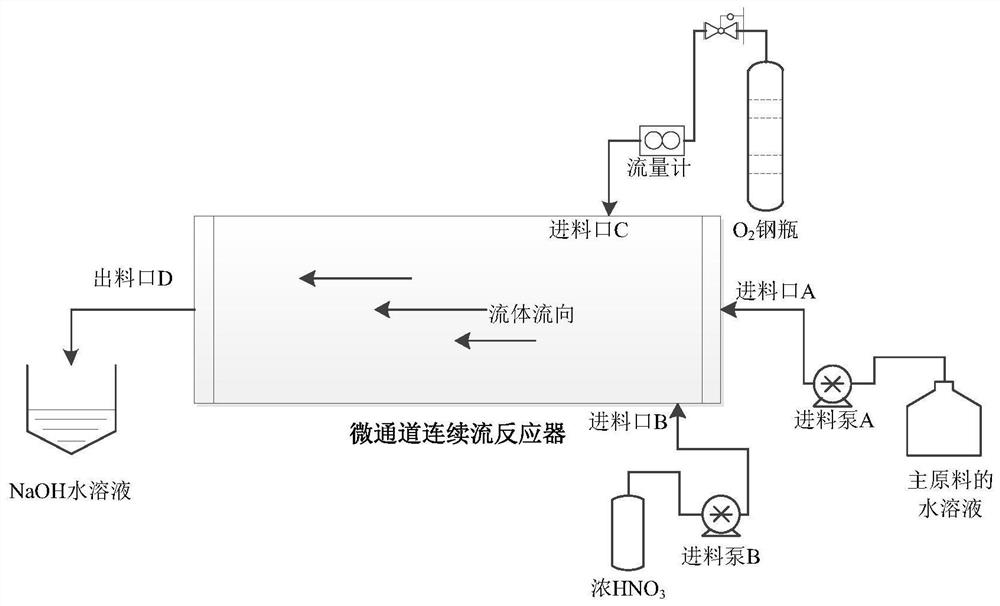
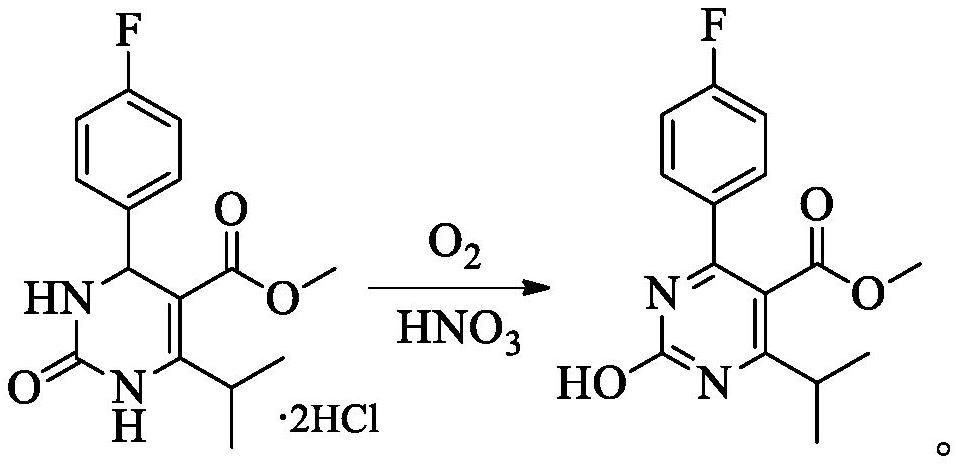
[0057] The microchannel reactor was cleaned with drinking water, and the temperature of the temperature control device of the reactor was set to 30°C, and the oxygen cylinder was connected to the inlet C of the microchannel reactor through a stainless steel pipeline for standby. Weigh 10g of 4-(4-fluorophenyl)-6-isopropyl-5-methoxycarbonyl-3,4-dihydropyrimidin-2(1H)-one dihydrochloride (27.4mmol) into 100mL beaker A, then add 50mL drinking water to beaker A, stir to dissolve, set aside. Weigh 0.9g of 68% concentrated nitric acid (9.6mmol, 0.35eq) into a 10mL graduated test tube B, add water to dilute to 10mL, and set aside. Prepare 30mL of 10% sodium hydroxide solution, pour it into beaker D, put a magnetic stirrer in beaker D and place it on the magnetic stirrer, connect the pipe at outlet D to the top of beaker D, and set aside.
[0058] Turn on feeding pump A, set the flow rate of feedi...
Embodiment 2
[0060] Embodiment 2: implement the technical scheme of the present invention in the traditional tank reactor
[0061] Add 10g of 4-(4-fluorophenyl)-6-isopropyl-5-methoxycarbonyl-3,4-dihydropyrimidin-2(1H)-one dihydrochloride successively to a 250mL three-necked flask (27.4mmol), 50mL of drinking water, stirring to dissolve. Under the condition of reaction temperature of 20-30°C, 0.9g of 68% concentrated nitric acid (9.6mmol, 0.35eq) was added. At the same time, oxygen was constantly fed into the reaction solution. After the reaction was stirred at 20-30° C. for 5 hours, 30 mL of 10% sodium hydroxide solution was added dropwise to the reaction solution. The obtained suspension was stirred for 20-30 minutes, filtered, and the filter cake was washed with 10 mL×2 drinking water. The wet cake was dried under reduced pressure at 50-60°C for 6 hours to obtain 8.33 g of white solid. The HPLC purity is 15.3%, and the main raw material remains 81.8%. A large amount of main raw mate...
Embodiment 3
[0062] Embodiment 3: the technical scheme disclosed in the patent CN200510106423.2 (HNO 3 :3.0eq)
[0063]
[0064]Add 10g (27.4mmol) 4-(4-fluorophenyl)-6-isopropyl-5-methoxycarbonyl-3,4-dihydropyrimidine-2( 1H)-Kone dihydrochloride and 5ml acetic acid. 7.6 g (82.2 mmol, 3.0 eq) of 68% nitric acid were slowly added to the mixture. A violent exotherm was observed during the dropwise addition. After the dropwise addition, 0.07 g (1 mmol) of sodium nitrite was added to the mixture, and the mixture was reacted at room temperature for 1 hour. After the reaction, gradually add 30% sodium hydroxide solution to the reaction solution to adjust the pH to 7-8, and stir the resulting suspension for 20-30 minutes. Filter and wash the filter cake with 10mL×2 drinking water. The wet cake was dried under reduced pressure at 50-60°C for 6 hours to obtain 6.88 g of white solid. The HPLC purity is 58.2%, and the main raw material remains 39.9%. A large amount of main raw materials have...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com