Synthesis method of hexafluorocyclotriphosphazene
A technology for hexafluorocyclotriphosphazene and hexachlorocyclotriphosphazene, which is applied in the field of synthesis of hexafluorocyclotriphosphazene, can solve the problems of long reaction time and high reaction temperature, and achieves short reaction time, high purity, and secondary The effect of less product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Put 104.3g of hexachlorocyclotriphosphazene, 113.3g of potassium fluoride, 0.011g of ionic liquid catalyst [Nbmm]OH, and 217.6g of anhydrous acetonitrile in a flask equipped with an electric stirrer, a thermometer, and a reflux condenser, at 30°C The reaction was carried out under the conditions, the reaction was closed after 2 hours, the filtrate was filtered, and the filtrate was rectified to obtain hexafluorocyclotriphosphazene with a yield of 98.7%.
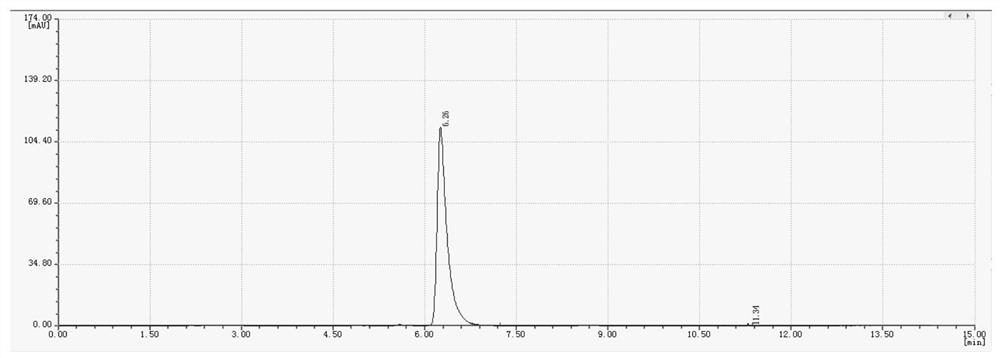
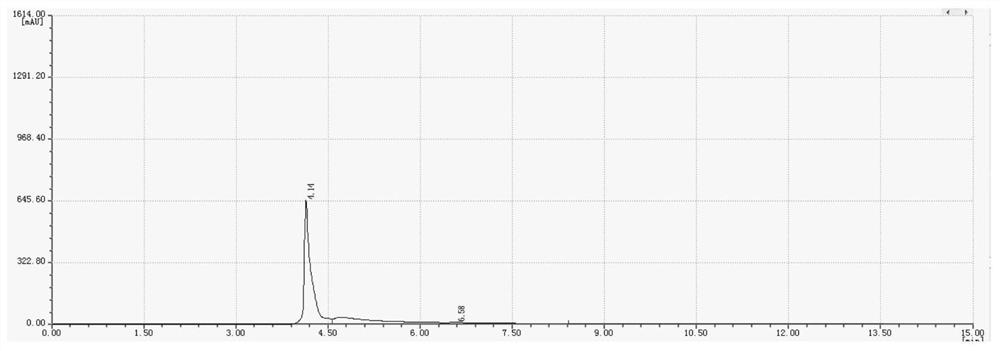
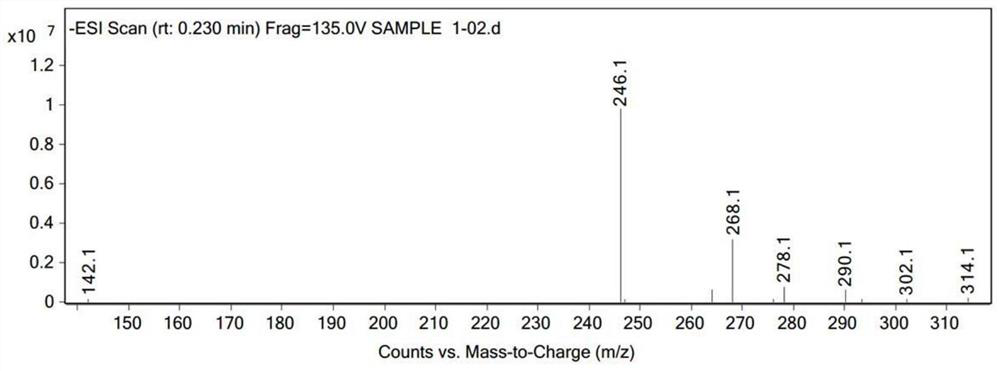
[0034] Wherein the liquid phase diagram of the hexachlorocyclotriphosphazene of raw material is as figure 1 As shown, the liquid phase diagram and mass spectrum of the product are shown as figure 2 with 3 As shown, it shows that hexafluorocyclotriphosphazene has been synthesized.
Embodiment 2
[0036] Put 104.3g of hexachlorocyclotriphosphazene, 113.3g of potassium fluoride, 0.011g of ionic liquid catalyst [Nbmm]OH, and 217.6g of anhydrous acetonitrile in a flask equipped with an electric stirrer, a thermometer, and a reflux condenser, at 50°C After 3 hours, the reaction was closed, the filtrate was filtered, and the filtrate was rectified to obtain hexafluorocyclotriphosphazene with a yield of 98.1%.
Embodiment 3
[0038] 104.3g of hexachlorocyclotriphosphazene, 113.3g of potassium fluoride, 0.011g of ionic liquid catalyst [Nbmm]OH, 217.6g of 1,4-dioxane are placed in a flask with an electric stirrer, a thermometer, and a reflux condenser reaction at 30° C., shut down the reaction after 2 hours, filter, and rectify the filtrate to obtain hexafluorocyclotriphosphazene with a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com