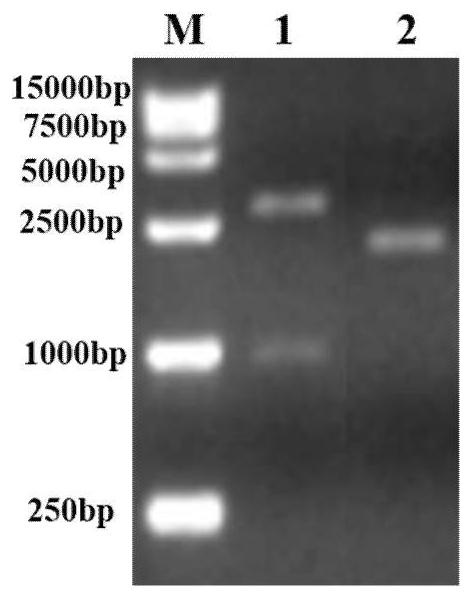
Respiratory syncytial virus full-length pre-fusion glycoprotein nucleotide sequence, recombinant adenovirus vector and application product thereof
A technology for fusing glycoprotein and nucleotide sequence, which is applied in the fields of fusion glycoprotein nucleotide sequence before full-length fusion of respiratory syncytial virus, recombinant adenovirus vector and its application products, and can solve the problem of low human infection rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The present invention provides a preparation method of recombinant adenovirus and a product containing the recombinant adenovirus, the active ingredient of which is the recombinant adenovirus obtained above, and the product can be a respiratory syncytial virus vaccine and be used for neutralizing respiratory syncytial virus Virus medicines, etc., for example, can be chimpanzee type 63 replication-deficient adenovirus vector vaccine carrying the prefusion glycoprotein of respiratory syncytial virus.
[0044] The preparation method of the recombinant adenovirus provided by the present invention comprises the steps of transfecting the recombinant adenovirus plasmid into the packaging cells, and then culturing the cells to obtain the adenovirus packaging cells, the adenovirus packaging cells have type 5 adenovirus E1 gene. The recombinant plasmid is obtained by inserting the specific DNA molecule into the orangutan type 63 replication defective adenovirus vector; the specifi...
Embodiment 1
[0045] Embodiment 1, the preparation of recombinant adenovirus
[0046] 1. Construction of shuttle plasmids (pShuttle63 and pShuttle26)
[0047] The artificially synthesized DNA sequence was inserted between the two Pac I restriction sites of the pShuttle-CMV vector, transformed into DH5α competent cells, and the plasmids were extracted to obtain the shuttle plasmids pShuttle63 and pShuttle26.
[0048] 2. Construction of backbone plasmids (pChAd63 and pAd26)
[0049] The E1 and E3 regions of the deleted adenovirus were obtained by restriction enzyme ligation and DNA Assembly methods, and E4orf6 was replaced by the backbone plasmids pChAd63 and pAd26 of human type 5 adenovirus E4orf6.
[0050] 3. Construction of shuttle plasmids carrying exogenous genes
[0051] 1. Design mDS-Cav1 site-directed mutagenesis primers, introduce the four mutation sites S155C, S190F, V207L and S290C into the full-length RSV F gene, phosphorylate the 5' end of the mutation primers, and carry out ...
Embodiment 2
[0070] Embodiment 2, the application of recombinant adenovirus
[0071] 1. Animal immunity
[0072] Female BALB / c mice aged 6-8 weeks were divided into 4 groups, 5 mice in each group, and were treated as follows:
[0073] The first group (G1 group): a single immunization was carried out by nasal drops, and the immune substance was rChAd63-empty virus solution (the virus amount was 1×10 10 VP);
[0074] The second group (G2 group): a single immunization was carried out by nasal drop method, and the immune substance was rChAd63-Fsyn virus liquid (the virus amount was 1×10 10 VP);
[0075] The third group (G3 group): a single immunization was carried out by nasal drops, and the immune substance was rChAd63-mDS-Cav1 virus solution (the virus amount was 1×10 10 VP);
[0076] The fourth group (G4 group): a single immunization was carried out by intramuscular injection, and the immune substance was FI-RSV virus liquid (the virus amount was 1.25×10 6 PFU);
[0077] The fifth gr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com