Method for synthesizing 6-chloro-1-hexanol by taking 1,6-hexanediol and cyanuric chloride as raw materials
A technology of cyanuric chloride and hexanediol, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxyl compounds, etc., can solve problems such as unsuitable for large-scale industrial production, large amount of acid waste water, and many impurities. Achieve the effect of being suitable for large-scale industrial application, mild reaction conditions, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
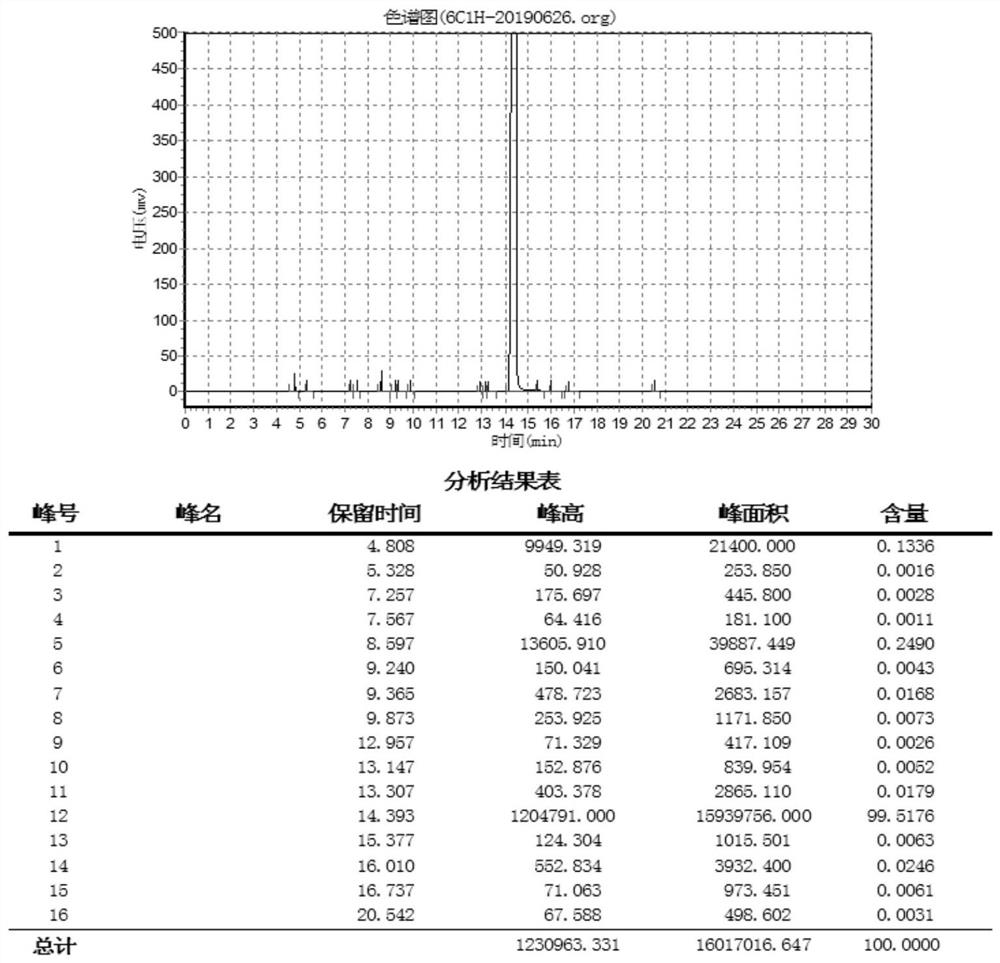
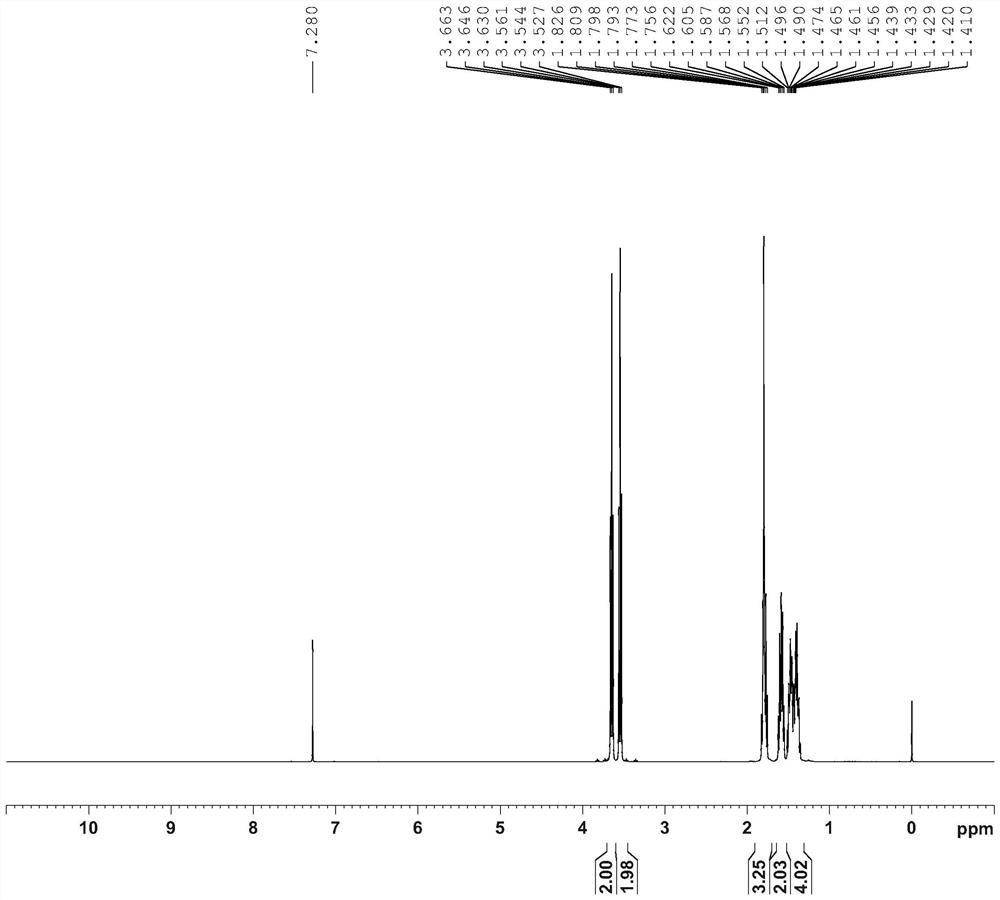
Image
Examples
Embodiment 1
[0041] 1) Add 3.7kg DMF to the four-necked bottle, stir, and control the temperature at 10-20°C, add 738g cyanuric chloride in batches, after the addition is complete, stir for 6 hours, and check that the reaction of cyanuric chloride has been completed.
[0042] 2) Prepare a mixed solution of 473g 1,6-hexanediol and 500g DMF, stir, control the temperature at -5-0°C, add the reaction solution in 1) dropwise, after the dropwise addition, stir at 0°C for reaction 2 After 1 hour, stop the refrigeration, raise the temperature naturally to 25°C, take a sample and control it, when the 1,6-hexanediol has completely reacted, the reaction is over.
[0043] 3) The mixed reaction solution obtained in step 3) was filtered under low vacuum, the filter cake was rinsed with 100 g of DMF, dried, and the DMF solution of the product was combined.
[0044] 4) Vacuum rectification product, control the vacuum degree at 5mmHg, slowly raise the temperature and rectify to obtain product: 520g, purity...
Embodiment 2
[0046] 1) Add 3.7kg dimethyl sulfoxide to the four-neck bottle, stir, and control the temperature at 10-20°C, add 738g cyanuric chloride in batches, after the addition is complete, stir for 2 hours, and check that the reaction of cyanuric chloride has been completed.
[0047]2) Prepare a mixed solution of 473g 1,6-hexanediol and 700g dimethyl sulfoxide, stir, control the temperature at 0-10°C, add the reaction solution prepared in 1) dropwise, after the dropwise addition is completed, put it under 10°C Stir the reaction for 2 hours, stop the refrigeration, naturally raise the temperature to 25°C, take a sample and control it, when the 1,6-hexanediol has completely reacted, the reaction is over.
[0048] 3) The mixed reaction solution obtained above was filtered under low vacuum, the filter cake was rinsed with 100 g of dimethyl sulfoxide, and dried, and the dimethyl sulfoxide solution of the product was combined.
[0049] 4) Vacuum distillation product, control the vacuum degr...
Embodiment 3
[0051] 1) Add 15kg of N,N-xylaniline into the 20L reactor, stir, control the temperature at 10-20°C, add 3.1kg of cyanuric chloride in batches, after adding, stir for 6 hours, and check that the cyanuric chloride has reacted complete.
[0052] 2) Prepare a mixed solution of 1.9kg 1,6-hexanediol and 2kg of dichloromethane, stir, control the temperature at -5-0°C, add the reaction solution prepared in 1) dropwise, after the dropwise addition is completed, set the temperature at 0°C Stir the reaction for 2 hours, stop the refrigeration, naturally rise to 25°C, take a sample and control it, when the 1,6-hexanediol has completely reacted, the reaction is over;
[0053] 3) The mixed reaction solution obtained above was filtered under low vacuum, the filter cake was rinsed with 500 g of dichloromethane, drained, and the filtrates were combined.
[0054] 4) Atmospheric rectification to recover dichloromethane. After the recovery of dichloromethane, control the vacuum at 3mmHg, slowly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com