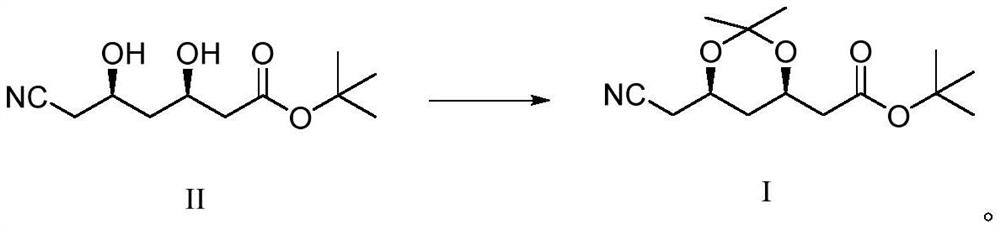
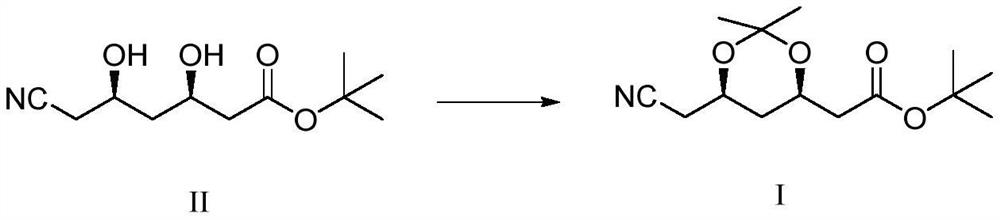
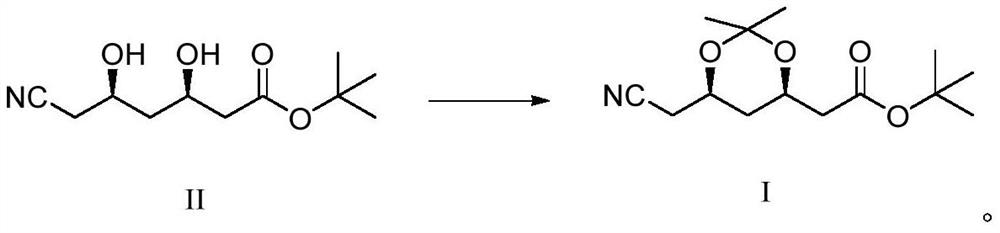
Synthetic method of atorvastatin calcium intermediate
A technology of atorvastatin calcium and synthesis method, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, etc., can solve the problem that the volume ratio of catalyst and raw material is too different , continuous operability is not strong, poor mixing effect and other problems, to achieve the effect of low production cost, short reaction time, high degree of automation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Under nitrogen protection, add 1000g of compound II and 700g of 2,2-dimethoxypropane into a 2L three-necked flask, start stirring, and cool down to about 10°C after compound II is dissolved. 75 g of Amberlite-15 acidic resin was filled in fixed bed reactor 1, and 75 g of Amberlite IRA-93 basic resin was filled in fixed bed reactor 2 . Use a metering pump to pump the mixture of compound II and 2,2-dimethoxypropane into the fixed-bed reactor 1 filled with acidic resin for catalytic reaction, the reaction temperature is 15°C, and the time is 40s. After the reaction is completed, the The obtained reaction liquid was pumped into the fixed-bed reactor 2 filled with basic resin for quenching, the quenching temperature was 15°C, and the quenching time was 40s. During the reaction, take a sample of the TLC control (take the reaction solution, add a small amount of triethylamine to adjust the pH to alkaline, spot the plate, KMnO 4 Color development), when compound II disappears ...
Embodiment 2
[0025] Under the protection of nitrogen, add 1000g of compound II and 900g of 2,2-dimethoxypropane into a 2L three-necked flask, start stirring, and cool down to about 10°C after compound II is dissolved. Fixed-bed reactor 1 was filled with 40 g of Amberlite-15 acidic resin, and fixed-bed reactor 2 was filled with 40 g of Amberlite IRA-93 basic resin. Use a metering pump to pump the mixture of compound II and 2,2-dimethoxypropane into the fixed-bed reactor 1 filled with acidic resin for catalytic reaction, the reaction temperature is 25°C, and the time is 20s. After the reaction is completed, the The obtained reaction liquid was pumped into the fixed-bed reactor 2 filled with basic resin for quenching, the quenching temperature was 25°C, and the quenching time was 20s. During the reaction, take a sample of the TLC control (take the reaction solution, add a small amount of triethylamine to adjust the pH to alkaline, spot the plate, KMnO 4 Color development), when compound II d...
Embodiment 3
[0027]Under the protection of nitrogen, add 1000g of compound II and 500g of 2,2-dimethoxypropane into a 2L three-necked flask, start stirring, and cool down to about 10°C after compound II is dissolved. Fixed-bed reactor 1 was filled with 90 g of Amberlite-15 acidic resin, and fixed-bed reactor 2 was filled with 90 g of Amberlite IRA-93 basic resin. Use a metering pump to pump the mixed solution of compound II and 2,2-dimethoxypropane into the fixed bed reactor 1 filled with acidic resin to carry out the catalytic reaction, the reaction temperature is 5°C, and the time is 60s. After the reaction is completed, the The obtained reaction solution was pumped into the fixed-bed reactor 2 filled with basic resin for quenching, the quenching temperature was 5°C, and the quenching time was 60s. During the reaction, take a sample of the TLC control (take the reaction solution, add a small amount of triethylamine to adjust the pH to alkaline, spot the plate, KMnO 4 Color development),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com