A camptothecin-based dimer compound, its preparation and application
A technology of dimer and camptothecin, applied in the field of medicine, can solve the problems of difficult systemic drug administration, many adverse reactions, hindering application, etc., and achieve the effects of enhancing solubility, reducing toxicity, and reducing tumor weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The present invention also provides a method for preparing the dimer compound, comprising the following steps:
[0044] (1) The hydroxyl group in the camptothecin structural formula is converted into a carbonyl chloride compound by a substitution reaction; then the carbonyl chloride compound is esterified with an alkyl diol containing a disulfide bond, so that the camptothecin and the disulfide-containing The alkyl diols of bonds are connected by carbonate bonds to obtain intermediate products;
[0045] (2) After the intermediate product described in step (1) is separated and purified, the hydroxyl contained in its structure undergoes a substitution reaction and is converted into an activated product, which is a carbonyl chloride compound; then the carbonyl chloride compound is substituted with NLG919 Reaction, so that the intermediate product and NLG919 are connected through a carbonate bond, and the obtained product is separated and purified to obtain the dimer compou...
Embodiment 1
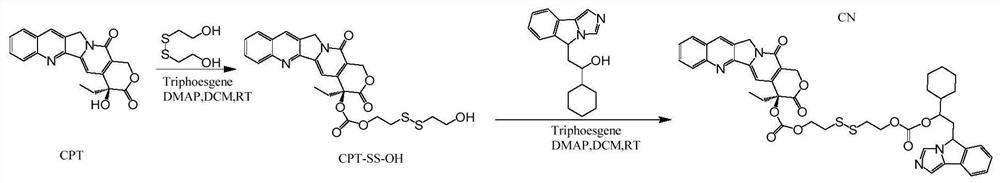
[0060] as per figure 1 The preparation of the compound CN shown in the formula (2) according to the schematic flow diagram is carried out according to the following steps:
[0061] (1) Reaction of camptothecin with 2,2'-dithiodiethanol: dissolve camptothecin (1mmol, 348.34mg), triphosgene (1 / 3mmol, 98.91mg) in 20ml of dichloromethane, add 4-di Aminopyridine (1 mmol, 122.17 mg) was reacted on ice in the dark for 30 minutes, and 2,2'-dithiodiethanol (1 mmol, 154.25 mg) was added to react at room temperature in the dark for 12 h.
[0062] (2) Extract the product obtained in (1) with 0.1M hydrochloric acid aqueous solution, saturated saline, and ultrapure water, separate the organic phase, and dry in a vacuum oven at 37°C to remove the organic solvent and dry.
[0063] (3) Dissolve the product (1eq) and triphosgene (1 / 3eq) obtained in (2) in dichloromethane, add 4-dimethylaminopyridine (1eq) and react for 30 minutes, then add NLG919 (1eq) to react.
[0064] (4) Extract the produ...
Embodiment 2
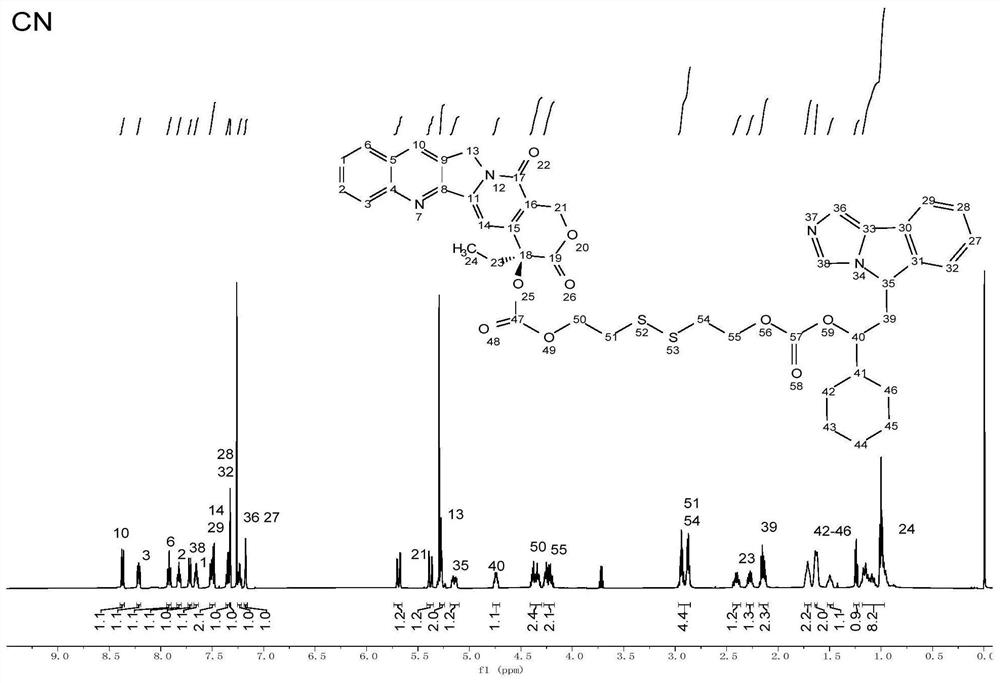
[0067] Reduction-responsive studies of compounds.
[0068] Take a small amount of compound CN in 100 mM GSH aqueous solution and stir for 12 h. The stirred solution was analyzed by mass spectrometry, and the mass spectrometry results were as follows: Figure 4 shown. The results of mass spectrometry showed that compound CN could be degraded to the original camptothecin and NLG919 under reducing conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com