Whole-cell vaccine, and preparation method and application thereof
A whole-cell vaccine and single-cell technology, which can be applied to pharmaceutical formulations, medical preparations containing active ingredients, antibody medical ingredients, etc., can solve the problems of inability to perform specific identification and insufficient whole-cell vaccine antigens, and achieve enhanced application effects And practicality, improve the effect of wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The second aspect of the embodiment of the present application provides a method for preparing a whole cell vaccine, comprising the following steps:
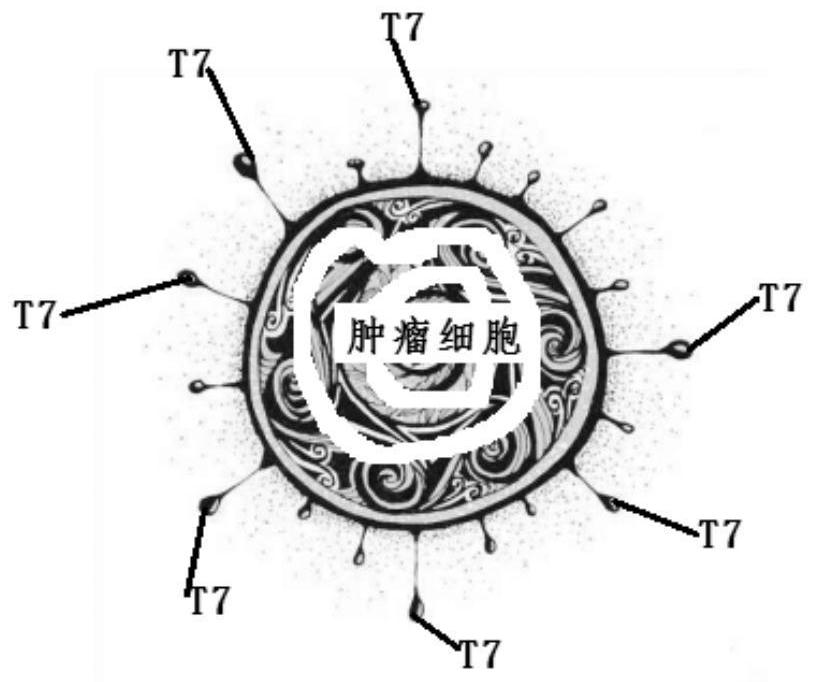
[0048] S01. According to the whole cell vaccine, tumor cells, TLR7 small molecule agonist molecules and conjugated chain molecules are provided;
[0049] S02. Use the activated ester method to esterify the TLR7 small molecule agonist molecule and the coupled chain molecule to obtain the TLR7 small molecule agonist-coupled chain activated ester molecule or use the TLR7 small molecule agonist with NCS functional groups; tumor cells Perform digestion to obtain tumor single cells;
[0050] S03. Mix TLR7 small molecule agonist-conjugated chain activated ester molecule or TLR7 small molecule agonist with NCS functional group and tumor single cells to obtain coupled tumor single cells;
[0051] S04. Inactivate the coupled tumor single cells to obtain a whole cell vaccine.
[0052] The preparation method of the whole cell vacci...
Embodiment 1
[0070] A kind of whole cell vaccine and preparation method thereof
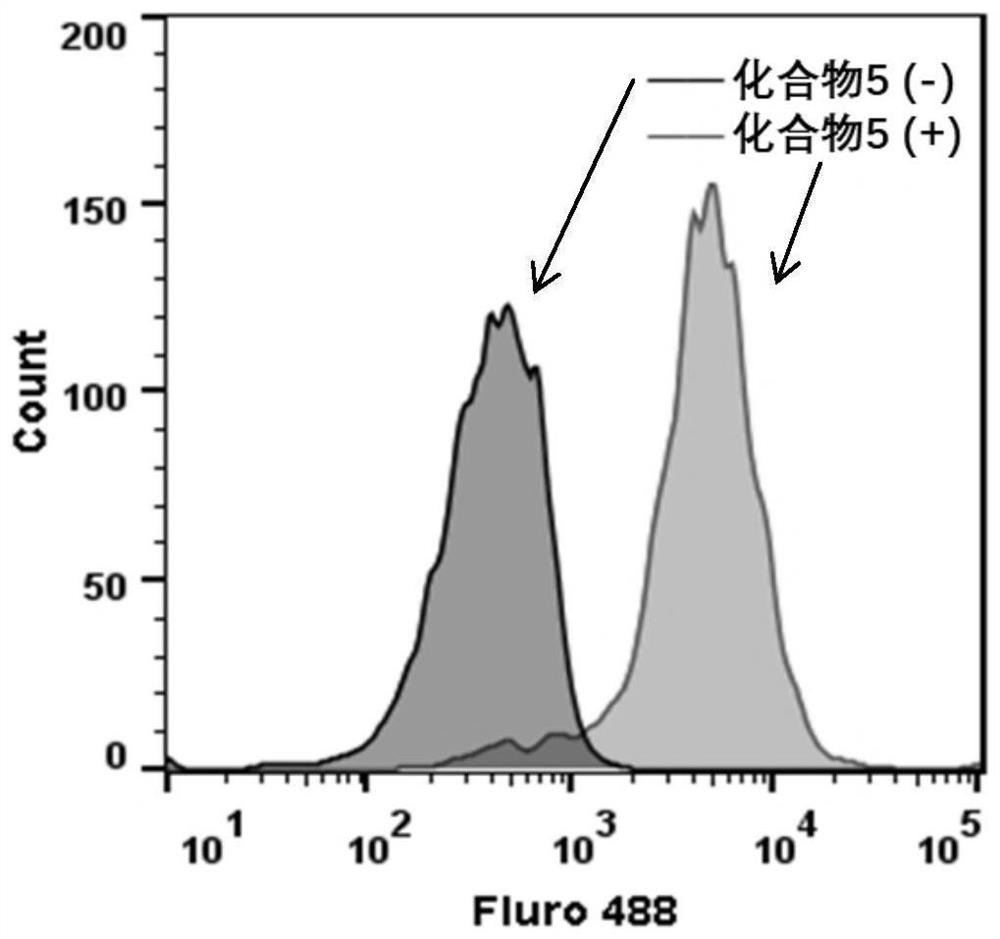
[0071] The whole cell vaccine includes: tumor cells, TLR7 small molecule agonist molecule compound 5, the structural formula is as follows: And the coupled chain molecule 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride.
[0072] The preparation method comprises the following steps:
[0073] (1) Provide tumor cells, TLR7 small molecule agonist molecules and conjugated chain molecules;
[0074] (2) Esterifying the TLR7 small-molecule agonist molecule and the conjugated chain molecule by an activated ester method to obtain the TLR7 small-molecule agonist-conjugated chain molecule;
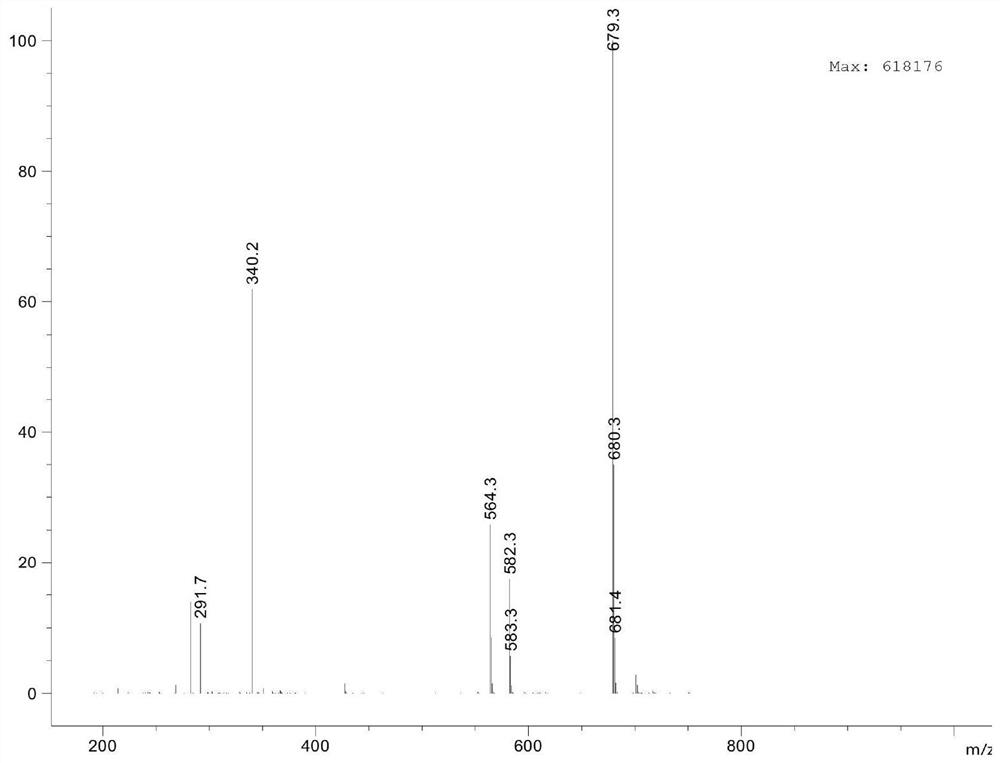
[0075] Specifically: Weigh 0.97mg TLR7 small molecule agonist compound 5, 0.25mg N-hydroxysuccinimide (NHS) and 0.38mg 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride Salt (EDCI) was dissolved in 166 μL of dimethyl sulfoxide (DMSO), and the reaction was shaken at room temperature. The reaction formula is sho...
Embodiment 2
[0083] A kind of whole cell vaccine and preparation method thereof
[0084] The whole cell vaccine includes: tumor cells, TLR7 small molecule agonist molecule compound 2, the structural formula is as follows: The preparation method comprises the following steps:
[0085] (1) Provide tumor cells, TLR7 small molecule agonist molecule 2;
[0086] (2) The TLR7 small molecule agonist molecule 2 and tumor cells are directly subjected to thiourea reaction by using the isothiocyanate method;
[0087] (3) providing mouse breast cancer 4T1 cells as tumor cells, digesting 4T1 cells with trypsin, washing with PBS twice, and counting to obtain 4T1 tumor single cells;
[0088] (4) Mixing TLR7 small molecule agonist molecule 2 and tumor single cells to obtain coupled tumor single cells;
[0089] Specifically: at a cell concentration of 3 x 10 7 A DMSO solution (10 mM) of TLR7 small-molecule agonist Compound 2 (10 mM) was added to 4T1 tumor single cells per ml to a final concentration of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com