Method for extracting lithium from salt lake brine
A salt lake brine and extraction technology, applied in the field of lithium extraction, can solve the problems of potential safety hazards, large temperature difference changes, and high equipment costs, and achieve the effects of avoiding acid corrosion, low production costs, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
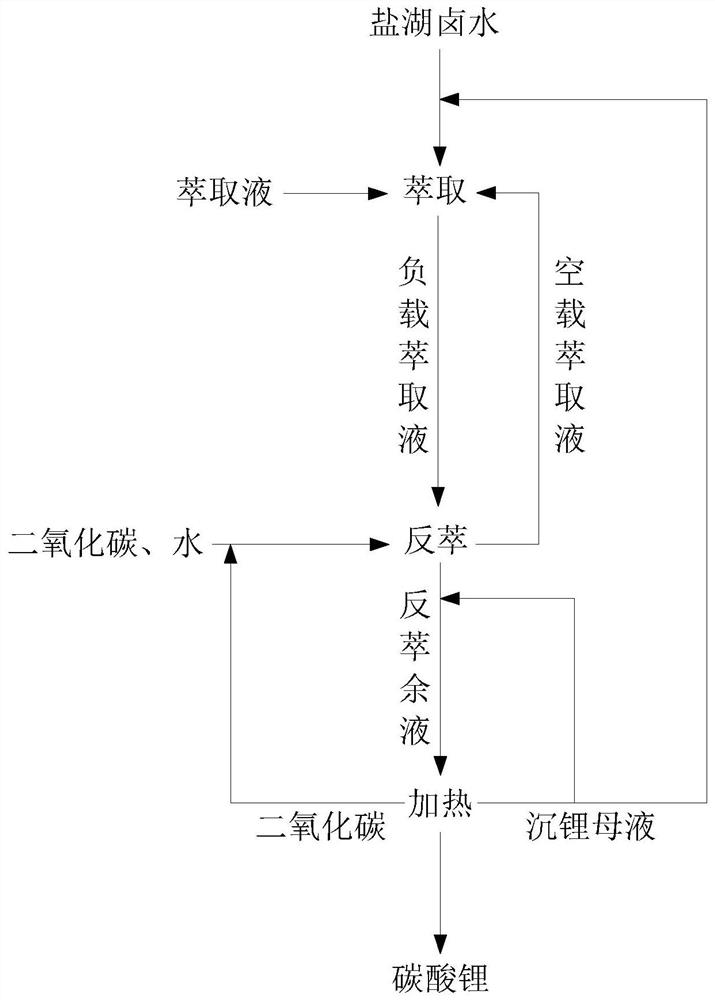
[0072] First take 20mL of benzoyltrifluoroacetone, 20mL of tris(2-ethylhexyl) phosphate, and 60mL of kerosene in a separatory funnel and mix them evenly to obtain the extract, then add alkaline salt lake brine containing 0.26g / L lithium 300mL, no need to adjust the pH value of the brine, shake and extract for 5 minutes, separate the aqueous phase and the loaded extract, extract three times, and combine the loaded extract. Wherein, the pH of the loading extract is 9.
[0073] Mix carbon dioxide and pure water with the above-mentioned loaded extract, and back-extract three times. The single back-extraction time is 5 minutes. The temperature when carbon dioxide is introduced is 25°C, and the pressure is 0.1MPa. It is 1:3. The aqueous phase is collected to obtain the lithium bicarbonate-containing back raffinate, the pH of the back raffinate is 9, and the empty extract is recycled to the extraction stage for further use.
[0074] The aqueous solution of lithium bicarbonate colle...
Embodiment 2
[0077] Take 5mL of benzoyltrifluoroacetone, 5mL of trihexyl phosphate, and 15mL of kerosene in a separating funnel and mix them evenly to obtain the extract, then add 100mL of alkaline salt lake brine containing 2.2g / L lithium, without adjusting the pH of the brine The value was 11, and the aqueous phase and the loaded extract were separated after 10 minutes of shaking extraction, extracted three times, and the loaded extract was combined. Wherein, the pH of the loading extract is 11.
[0078] Mix carbon dioxide and pure water with the above-mentioned loaded extract, and back-extract three times. The single back-extraction time is 5 minutes. The temperature when carbon dioxide is introduced is 20°C, and the pressure is 0.15MPa. It is 1:2. The aqueous phase is collected to obtain the lithium bicarbonate-containing back raffinate, the pH of the back raffinate is 10, and the unloaded extract is recycled to the extraction stage for further use.
[0079] The aqueous solution of l...
Embodiment 3
[0082] Take 75mL of benzoyl trifluorodecanone, 75mL of tripentyl phosphate, and 200mL of kerosene in a separatory funnel and mix them evenly to obtain the extract, then add 700mL of alkaline salt lake brine containing 1.5g / L lithium, no need to adjust The pH value of the brine was shaken and extracted for 10 minutes, and the aqueous phase and the loaded extract were separated, extracted three times, and the loaded extract was combined. Wherein, the pH of the loading extract is 10.
[0083]Mix carbon dioxide and pure water with the above-mentioned loaded extract, and back-extract three times. The single-time back-extraction time is 6 minutes. The temperature when carbon dioxide is introduced is 30°C and the pressure is 0.2MPa. 1:1. The aqueous phase is collected to obtain the lithium bicarbonate-containing back raffinate, the pH of the back raffinate is 10, and the unloaded extract is recycled to the extraction stage for further use.
[0084] The aqueous solution of lithium b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com