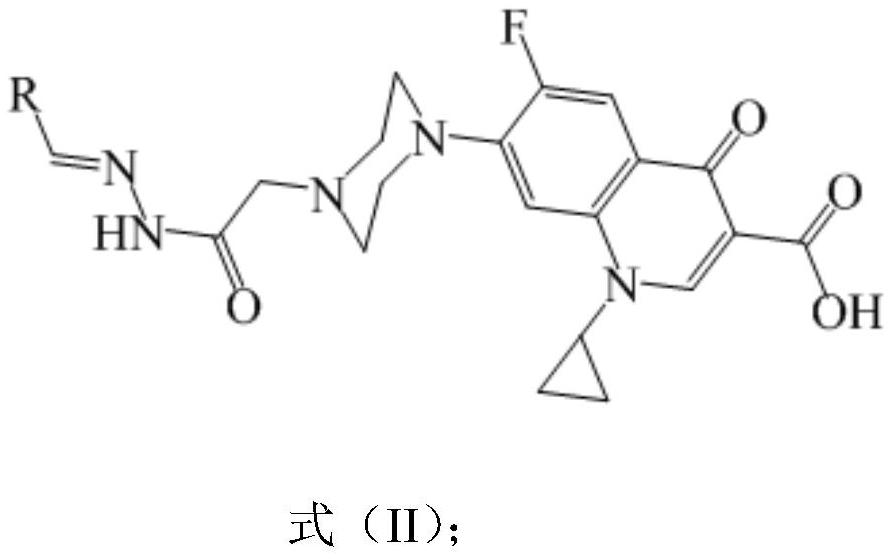
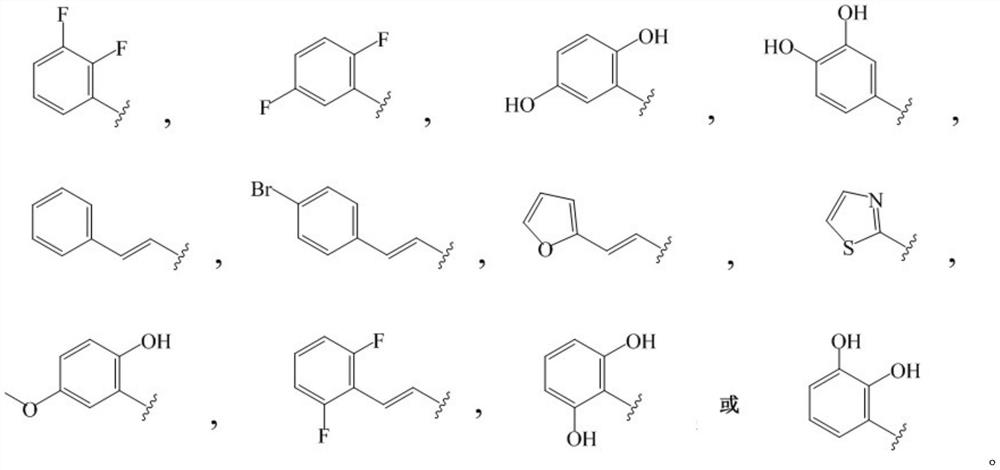
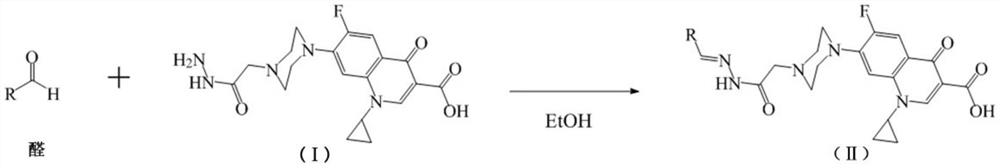
N-acylhydrazone-ciprofloxacin heterozygote and preparation method and application thereof
A technology of ciprofloxacin and acyl hydrazone, which is applied in the field of antibacterial and can solve the problems of increasing bacterial resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (E)-1-cyclopropyl-6-fluoro-7-(4-(2-(2,3-difluorobenzylidene)hydrazino-2-oxyethyl)piperazin-1-yl) -4-Oxygen-1,4-dihydroquinoline-3-carboxylic acid (IIa) preparation steps:
[0024]
[0025] Add 5mmol ciprofloxacin, 5mmol ethyl chloroacetate, 50mL N,N-dimethylformamide (DMF), 0.1mol triethylamine into a 100mL flask, stir and react at 100°C for 24 hours, after the reaction is complete, depressurize Most of the solvent was removed by rotary evaporation, cooled, filtered, and the filter residue was washed with methanol and filtered to obtain the precursor compound. Add 2.5mmol of precursor compound, 12.5mmol of hydrazine hydrate, and 10mL of absolute ethanol into a 100mL flask, stir and react at 75°C for 20 hours, cool, filter, wash with ethanol, and air-dry to obtain intermediate I, namely 1-cyclopropyl- 6-fluoro-7-(4-(2-hydrazino-2-oxyethyl)piperazin-1-yl)-4-oxy-1,4-dihydroquinoline-3-carboxylic acid.
[0026] 1.0 mmol of intermediate I (1-cyclopropyl-6-fluoro-7-(4-(2...
Embodiment 2
[0028] (E)-1-cyclopropyl-6-fluoro-7-(4-(2-(2,5-difluorobenzylidene)hydrazino-2-oxyethyl)piperazin-1-yl) -4-Oxygen-1,4-dihydroquinoline-3-carboxylic acid (IIb) preparation steps:
[0029]
[0030] Add 5mmol ciprofloxacin, 5mmol ethyl bromoacetate, 50mL N,N-dimethylformamide (DMF), 0.1mol triethylamine into a 100mL flask, stir and react at 100°C for 24 hours, after the reaction is complete, depressurize Most of the solvent was removed by rotary evaporation, cooled, filtered, and the filter residue was washed with methanol and filtered to obtain the precursor compound. Add 2.5mmol of precursor compound, 12.5mmol of hydrazine hydrate, and 10mL of absolute ethanol into a 100mL flask, stir and react at 75°C for 20 hours, cool, filter, wash with ethanol, and air-dry to obtain intermediate I, namely 1-cyclopropyl- 6-fluoro-7-(4-(2-hydrazino-2-oxyethyl)piperazin-1-yl)-4-oxy-1,4-dihydroquinoline-3-carboxylic acid.
[0031] 1.0 mmol of intermediate I (1-cyclopropyl-6-fluoro-7-(4-(2-...
Embodiment 3
[0033] (E)-1-cyclopropyl-6-fluoro-7-(4-(2-((1E,2E)-3-(furan-2-yl)allylidene)hydrazino-2-oxyl group Preparation steps of ethyl) piperazin-1-yl)-4-oxyl group-1,4-dihydroquinoline-3-carboxylic acid (IIc):
[0034]
[0035] 1.0 mmol of intermediate I (1-cyclopropyl-6-fluoro-7-(4-(2-hydrazino-2-oxyethyl)piperazin-1-yl)-4-oxyl-1 , 4-dihydroquinoline-3-carboxylic acid), 2.0mmol (E)-3-(furan-2-yl)acrolein, and 20mL of absolute ethanol were added to a 100mL flask, stirred and reacted at 60°C for 24 hours, and the reaction After completion, filter, wash with ethanol, and air-dry to obtain the target product (IIc) with a yield of 80%.1 H NMR(400MHz,DMSO)δ15.15(s,1H,-COOH),11.21 and 11.15(s,1H,-NH-CO-is affected by keto-form and enol-form),8.61(s,1H, quinoline),8.06,8.03and 7.75(s,1H,-CH=N-is affected by keto-form and enol-form),7.82 and7.79(d,1H,quinoline),7.75,7.73 and 7.71(m, 1H,furyl),7.54 and 7.52(d,1H,quinoline),6.86(t,1H,furyl),6.69-6.57(m,3H,furyl and allylidene),3.80(s,1H,cy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com