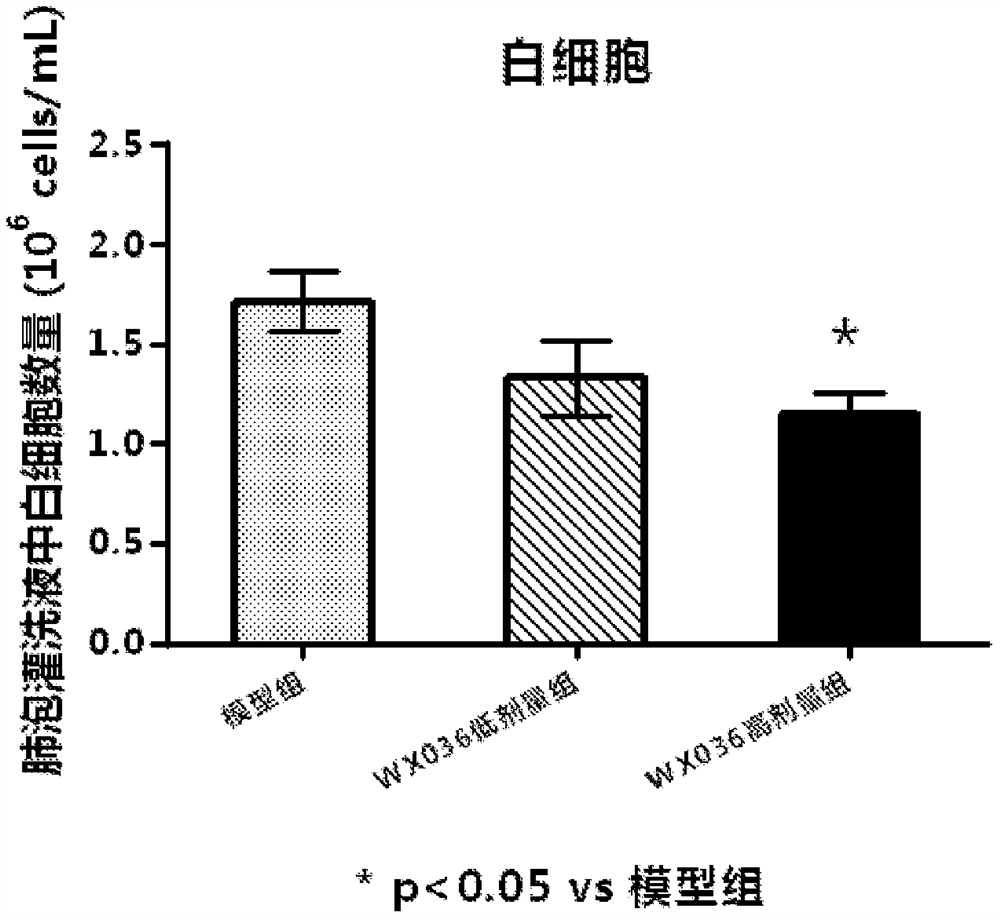
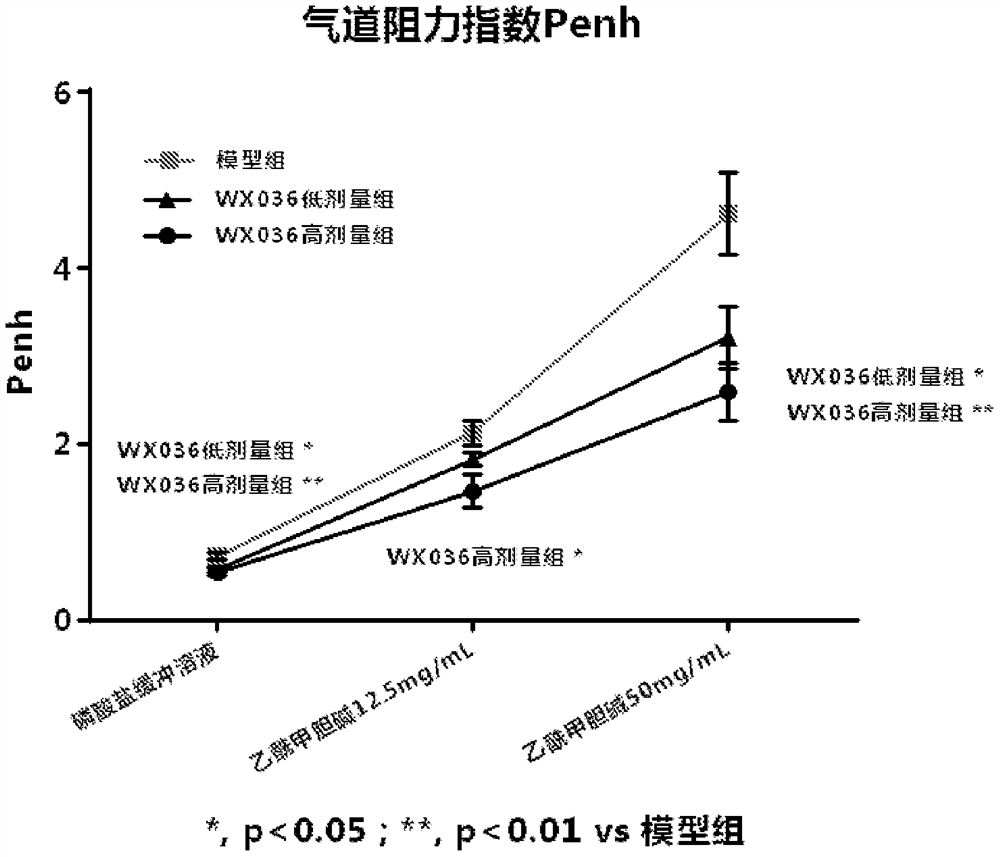
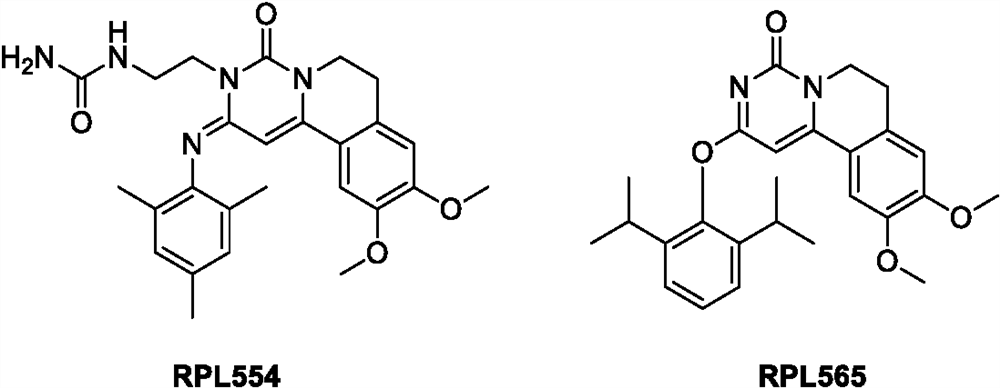
Triadyncyclic compounds as dual pde3/pde4 inhibitors
A compound and heterocyclyl technology, applied in the field of chronic obstructive pulmonary disease, can solve the problems of unsatisfactory anti-inflammatory effect and unsatisfactory PDE4 inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Embodiment 1: the synthesis of compound BB-1
[0144]
[0145] Step 1: Synthesis of compound BB-1-2
[0146] Under nitrogen atmosphere, a mixture of compound BB-1-1 (21.10 g) and ethyl cyanoacetate (11.00 g, 10.38 mL) was stirred at 100° C. for 16 hours. After the reaction was completed, the mixture was cooled to 70° C., and ethanol (30 mL) was slowly added dropwise, and a large amount of solids precipitated out. After filtration, the filter cake was dried under reduced pressure to obtain the product BB-1-2.
[0147] 1 H NMR (400MHz, DMSO-d6) δ=8.26(t, J=5.2Hz, 1H), 6.86(d, J=8.0Hz, 1H), 6.79(br s, 1H), 6.71(d, 8.0Hz, 1H), 4.00(q, J=6.8Hz, 2H), 3.72(s, 3H), 3.59(s, 2H), 3.31-3.23(m, 2H), 2.64(t, J=7.2Hz, 2H), 1.32(t, J=6.8Hz, 3H). MS-ESI m / z: 263.1[M+H] + .
[0148] Step 2: Synthesis of compound BB-1-3
[0149]Under nitrogen atmosphere, phosphorus oxychloride (379.50 g, 230.00 mL) was heated to 85° C., and compound BB-1-2 (26.00 g) was added in batches. The r...
Embodiment 2
[0162] Embodiment 2: the synthesis of compound BB-2
[0163]
[0164] Step 1: Synthesis of compound BB-2-2
[0165] Compound BB-2-1 (8g) and urea (9.18g) were dissolved in water (80mL) at room temperature, concentrated hydrochloric acid (12mol / L, 9.56mL) was added dropwise, heated to 110°C and stirred for 24 hours. After the reaction was completed, the reaction liquid was cooled to room temperature, filtered, and the filter cake was washed with water (50 mL x 2), and dried in vacuum to obtain compound BB-2-2.
[0166] 1 H NMR (400MHz, DMSO-d 6 )δ=6.84(br d, J=8.0Hz, 1H), 6.78(br s, 1H), 6.69(brd, J=8.0Hz, 1H), 6.02(brt, J=5.2Hz, 1H), 5.43( br s, 2H), 4.04-3.94(m, 2H), 3.71(s, 3H), 2.95(q, J=6.4Hz, 2H), 2.50-2.40(m, 2H), 1.66-1.59(m, 2H ), 1.35-1.25 (m, 3H). MS-ESI m / z: 253.2 [M+H] + .
[0167] Step 2: Synthesis of compound BB-2-3
[0168] Under a nitrogen atmosphere, sodium (6.38 g) was added to absolute ethanol (150 mL) in batches, and stirred until the sodium was c...
Embodiment 3
[0175] Embodiment 3: the synthesis of compound BB-3
[0176]
[0177] Step 1: Synthesis of compound BB-3-2
[0178] Compound BB-3-1 (2 g) was dissolved in 1,2-dichloroethane (20 mL) at room temperature, trimethylsilyl isocyanate (3.82 g) was added dropwise, and the temperature was raised to 90° C. and stirred for 18 hours. After the reaction was completed, the reaction solution was cooled to room temperature, concentrated under reduced pressure, and the obtained residue was slurried with methyl tert-butyl ether (10 mL) to obtain compound BB-3-2.
[0179] MS-ESI m / z: 255.1[M+H] + .
[0180] Step 2: Synthesis of compound BB-3-3
[0181]Under a nitrogen atmosphere, sodium (1.13 g) was added to absolute ethanol (85 mL) in batches, and stirred until the sodium was completely dissolved to obtain a fresh sodium ethoxide solution. Compound BB-3-2 (2.5 g) and diethyl malonate (3.94 g) were added thereto, followed by heating under reflux for 12 hours. The reaction solution was c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com