A kind of lentivirus stable packaging cell line and its preparation method
A technology for packaging cell lines and lentiviruses, which is applied in the fields of immunology and molecular biology, and can solve the problems of low lentivirus titer, many residual impurities, and unstable virus production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Construction of embodiment 1pPuro.coTetR plasmid, pVSVG plasmid and pGagPol-RRE-NES-cINT plasmid
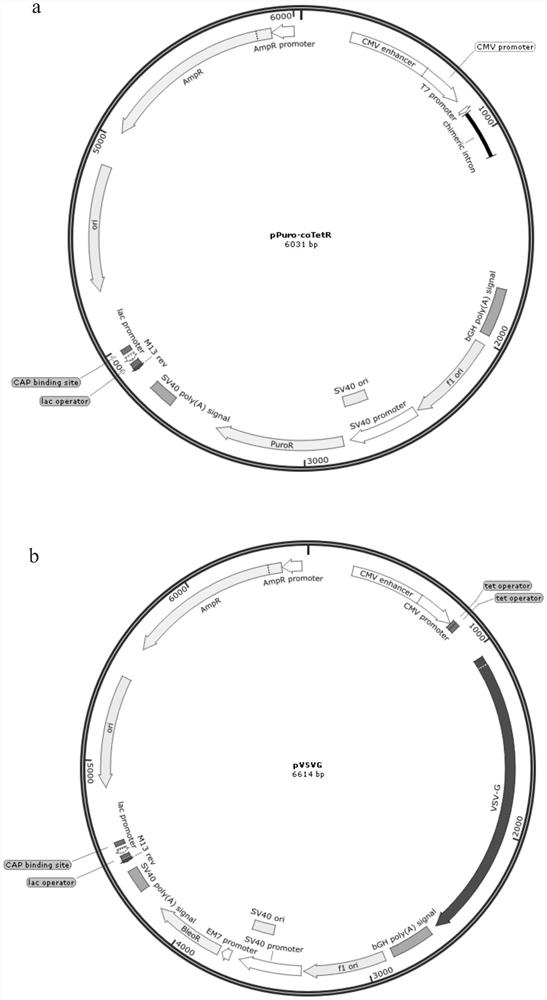
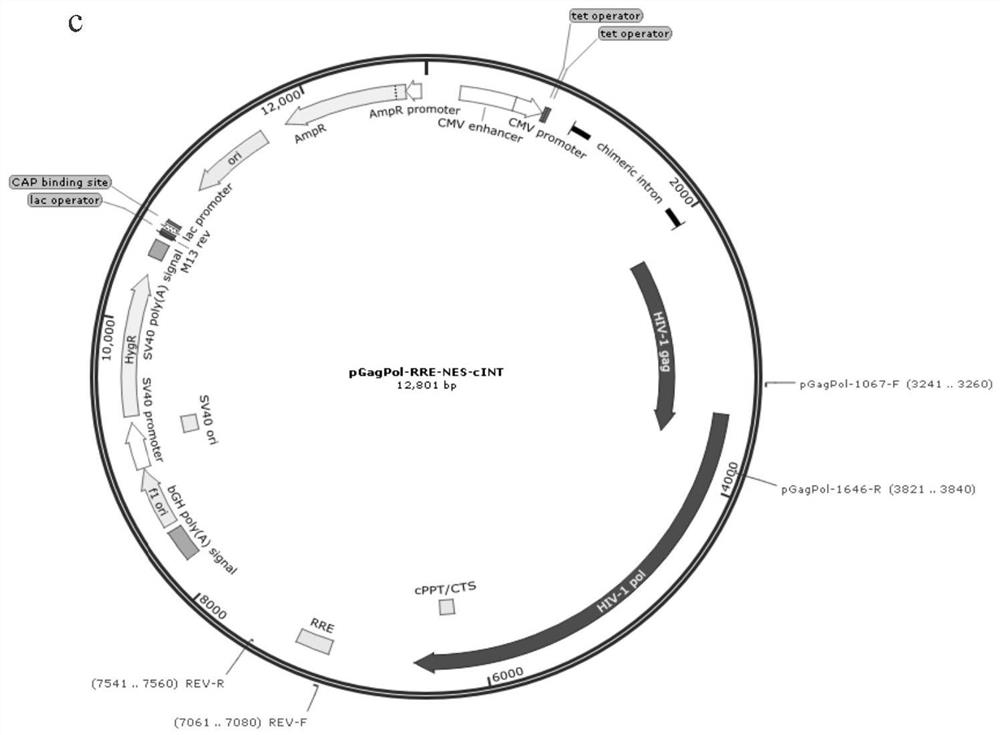
[0095] The lentivirus stable packaging cell line constructed in this example consists of three plasmids, pPuro.coTetR (comprising the CoTetR gene), pVSVG (comprising the VSVG gene), and pGagPol-RRE-NES-cINT (comprising the GagPol and Rev genes), which See the plasmid map of figure 1 a, figure 1 b and figure 1 c.
[0096] pPuro-coTetR vector construction:
[0097] The INT fragment (230bp) was obtained by primer TetR-WF-1F / TetR-1R-linker and template pIRESpuro3 (TaKaRa, product number: 631619), and by primer TetR-2F-linker / TetR-2R-linker and template coTetR (its sequence SEQ ID NO: 1) to obtain the coTetR fragment (624bp), through the primer TetR-3F-linker / TetR-WF-3R and the template pcDNA4 (Thermo, catalog number: V102020) to obtain the PA+F1 ori+SV40 promoter fragment (1042bp) . Then, by bridging PCR, the primer TetR-WF-1F / TetR-2R-linker was used to obtain the fragme...
Embodiment 2
[0108] Example 2 Preparation of lentivirus stable packaging cell line
[0109] The three plasmids constructed in Example 1 were linearized and digested with Fsp I (Thermo, catalog number: FD1224), and then dephosphorylated before being used to transfect 293 cells.
[0110] The linearized pPuro.coTetR and pVSVG plasmids were used to co-transfect 293 suspension cells with PEI (polyethyleneimine) (Polysciences, catalog number: 23966). 6 cells, PEI amount: (pPuro.coTetR plasmid amount+pVSVG plasmid amount)=4:1, after using 2 μg / mL Puro (puromycin) (InVivogen, product number: ant-pr-1), 400 μg / mL Zeo ( Bleomycin) (Invitrogen, product number: R25001) antibiotics were subjected to pressurized selection for 2 weeks to obtain drug-resistant positive stable cell pools, ie polyclonal PV cells. Polyclonal PV cells were plated in a 96-well plate (Thermo, catalog number: 167008) by limiting dilution method, with 200 μL of medium per well. Then observe under the microscope, pick out the we...
Embodiment 3
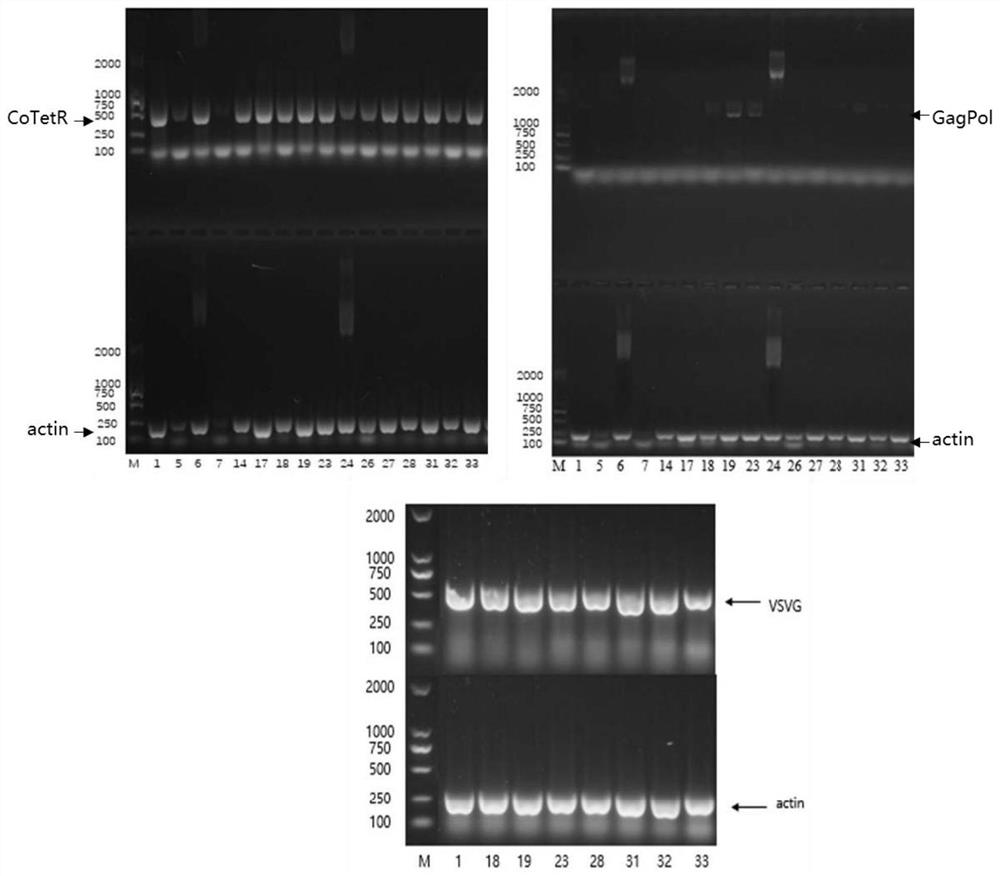
[0113] Example 3 Identification of stable packaging cell lines by semi-quantitative PCR
[0114] The surviving monoclonal cells in the 96-well plate were expanded and cultured, and 48-well plate, 24-well plate, 12-well plate, 6-well plate and shake flask E125 were used respectively. RNA was extracted from PVGR monoclonal cells, and after reverse transcription into cDNA, semi-quantitative PCR was performed to identify whether the target genes CoTetR, VSVG and GagPol were contained. β-actin was used as an internal reference gene. Specific steps are as follows:
[0115] The PVGR monoclonal cells were plated in a 12-well plate the day before, and 3 × 10 5 cells / mL, add 2 μg / mL dox (doxycycline) (Sigma, product number: D9891) on the second day, and harvest the cells on the third day for RNA extraction. with TRIzol TM Reagents (Invitrogen TM , Cat. No.: 15596026) to extract RNA, and the extraction method was operated according to the instructions.
[0116] The extracted RNA wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com