Medical application of cathepsin inhibitor
A technology of cathepsin and inhibitors, applied in the field of medicine, can solve the problems of not being able to meet the growing demand for medication of fibrosis patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Cathepsin Inhibitory Activity Test
[0034] 1. Experimental materials: cathepsin L and S at a temperature of 37°C, 5% CO 2 cultured in a humidified incubator. After each compound was dissolved in dimethyl sulfoxide (DMSO) under sterile conditions, it was diluted to the required concentration with RPMI-1640 culture solution, and the final concentration of DMSO was less than 0.5%. RPMI 1640 was purchased from Gibco (Grand Island, USA), fetal bovine serum (FBS) was purchased from Biological Industries, penicillin-streptomycin was purchased from HyClone Company, trypsin (Trypsin, 1:250) was purchased from Biosharp Company, dimethyl Sulfoxide (DMSO) was purchased from Sigma Chemical Company, and monodansylpentamethylenediamide (MDC) was purchased from Nanjing KGI Biotechnology Co., Ltd.
[0035] 2. Instruments: carbon dioxide incubator (SANYO, Japan, model: MCO-5AC), inverted microscope (OLYMPUS, Japan, model: CKX41), enzyme-linked immunoassay analyzer (Tecan, A...
Embodiment 2
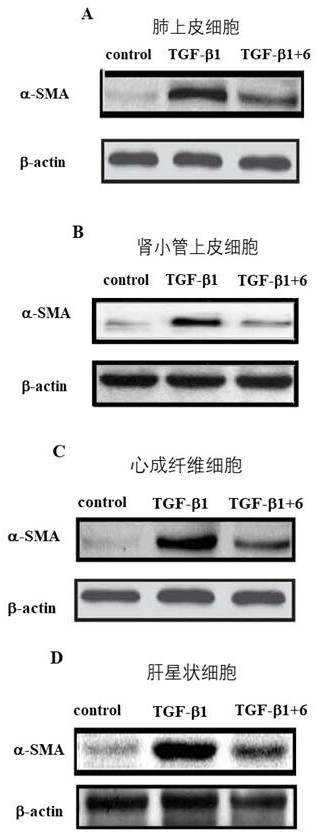
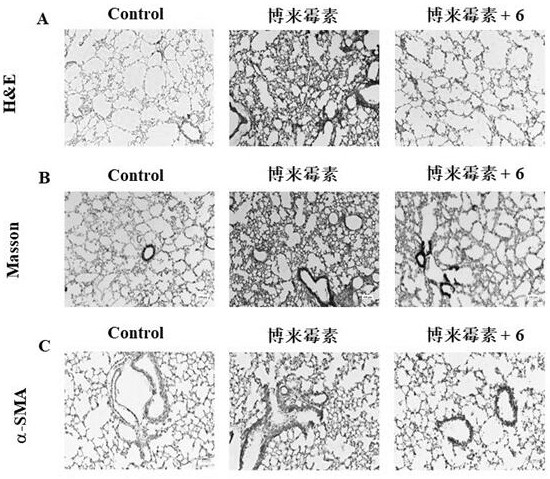
[0056] Example 2: Study on the anti-pulmonary fibrosis, liver fibrosis, kidney fibrosis and cardiac fibrosis of compound 6 based on the fibrosis marker protein α-SMA in vitro.
[0057] Take compound 6 with the best enzyme activity as an example:
[0058] Lung epithelial cells A549 cells, hepatic stellate cells LX-2, renal tubular epithelial cells CD3 and primary cardiac fibroblasts were pre-treated with 300 μM compound 6 or vehicle, and then stimulated by 10 ng / mL TGF-β1 to Myofibroblasts were transformed to construct lung, liver, kidney and heart fibrosis models in vitro, and the fibrosis marker protein α-SMA was used to characterize the degree of fibrosis. like figure 1 As shown, after 10ng / ml TGF-β1 stimulated human lung epithelial cells A549, hepatic stellate cells LX-2, renal tubular epithelial cells CD3 and primary mouse cardiac fibroblasts for 48h, the fibrosis marker protein α-SMA Significantly up-regulated (P<0.01=demonstrates successful construction of classic lung...
Embodiment 3
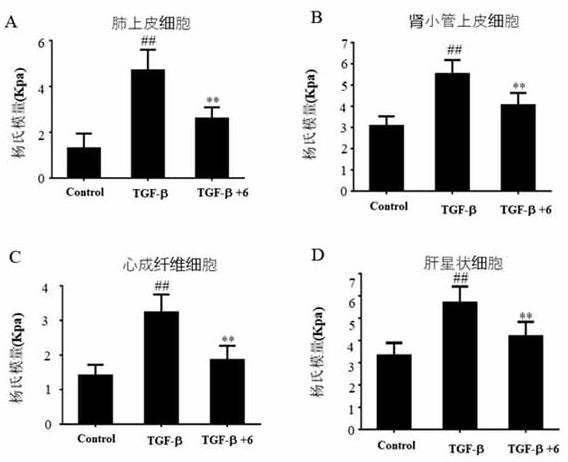
[0059] Example 3: Cell stiffness as a marker of fibrosis The effect of compound 6 on anti-pulmonary fibrosis, liver fibrosis, kidney fibrosis and cardiac fibrosis was investigated in vitro.
[0060] Previous studies have shown that cell stiffness can be used as a biomarker to characterize the degree of fibrosis. Lung epithelial cells A549 cells, hepatic stellate cells LX-2, renal tubular epithelial cells CD3 and primary cardiac fibroblasts were pre-treated with 300 μM compound 6 or vehicle, and then stimulated by 10 ng / mL TGF-β1 to Transformation of myofibroblasts to construct fibrosis models of lung, liver, kidney and heart in vitro. Atomic force microscopy was used to detect the changes in the stiffness of the above four cells after being stimulated by TGF-β1, and the cell stiffness (Young's modulus) was used as a marker to characterize degree of fibrosis. the result shows( figure 2 ), in the above models of pulmonary fibrosis, liver fibrosis, renal fibrosis and cardiac f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com