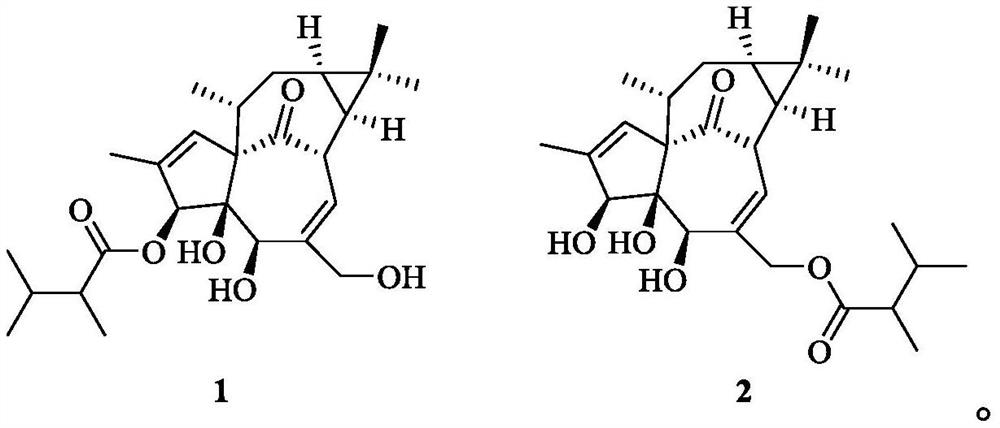
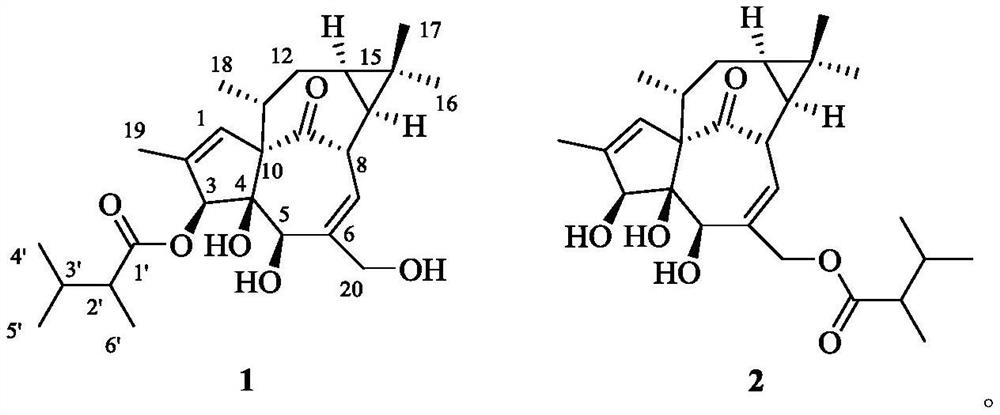
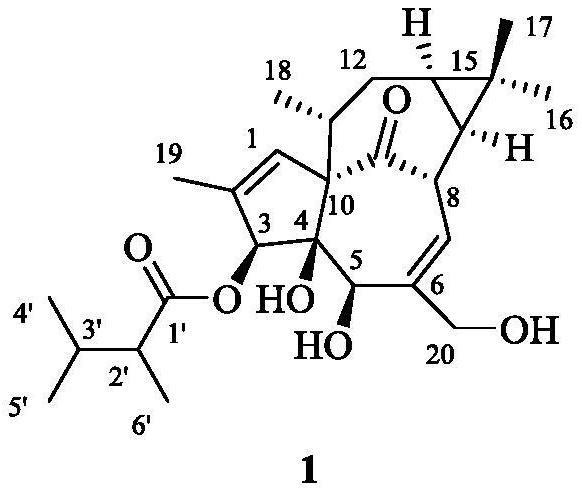
Kansuininol compounds as well as extraction method and application thereof
An ingenious diterpene and extraction method technology, which is applied in the application field of medicine, can solve the problems of low specific binding rate, strong toxic and side effects of human body, and limit clinical application, and achieve enhanced sensitivity, good safety, and good application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Under the room temperature condition, soak and extract the pulverized Gansui lump root (5kg) powder in 10L of 95% ethanol for 3 times, each time for 7 days, combine the extracts, and concentrate to obtain 491g of the extract. The kansui mentioned above was purchased from Bozhou in April 2017, and its origin is Houma, Shanxi. It was identified as Euphorbia kansui. The sample specimen was preserved by the Institute of Natural Medicine, School of Pharmacy, Zhejiang University of Technology, No. 20170422.
[0026] (2) the root extract 491g of Radix Kansui obtained by step (1), add 1L water for dissolving, extract twice with sherwood oil, each use 1L sherwood oil, concentrate the organic phase under reduced pressure, obtain organic phase (108g).
[0027] (3) Take the organic phase in step (2), adopt silica gel of 100-200 mesh silica gel as column chromatography packing column chromatography, column volume is 500mL, carry out gradient elution with sherwood oil / ethyl acetat...
Embodiment 2
[0039] Embodiment 2: compound reverses the tumor cell multidrug resistance effect of P-gp mediation
[0040] Compounds 1 and 2 prepared in Example 1 were formulated into 20 μM high-concentration solutions using cell culture medium, and their inhibitory effect on MCF-7 / Adr cell lines resistant to doxorubicin was evaluated. The results showed that after adding the above two compounds, , the number of cells in the drug-dosed group (compound 1 and 2) was 141% and 153% of the blank control, respectively, confirming that the two compounds had no obvious toxic activity on MCF-7 / Adr cells, which was similar to that of the short-chain branched chain in the literature. This coincides with the pro-proliferative effect of diterpenoids on cells at high concentrations.
[0041] MCF-7 / Adr cells with logarithmic growth were selected and seeded in a 96-well plate at a cell density of approximately 7×10 3 / well, the experimental group was compound (1 or 5 μM) combined with doxorubicin (0.25, 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com