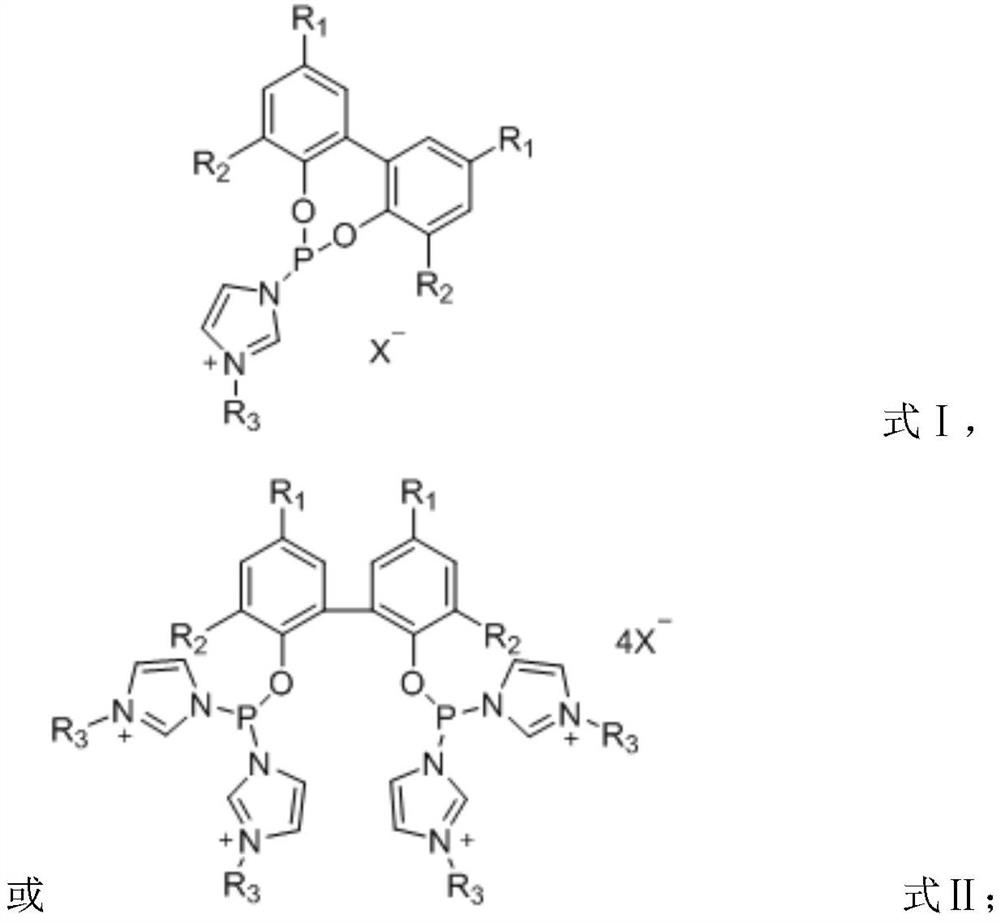
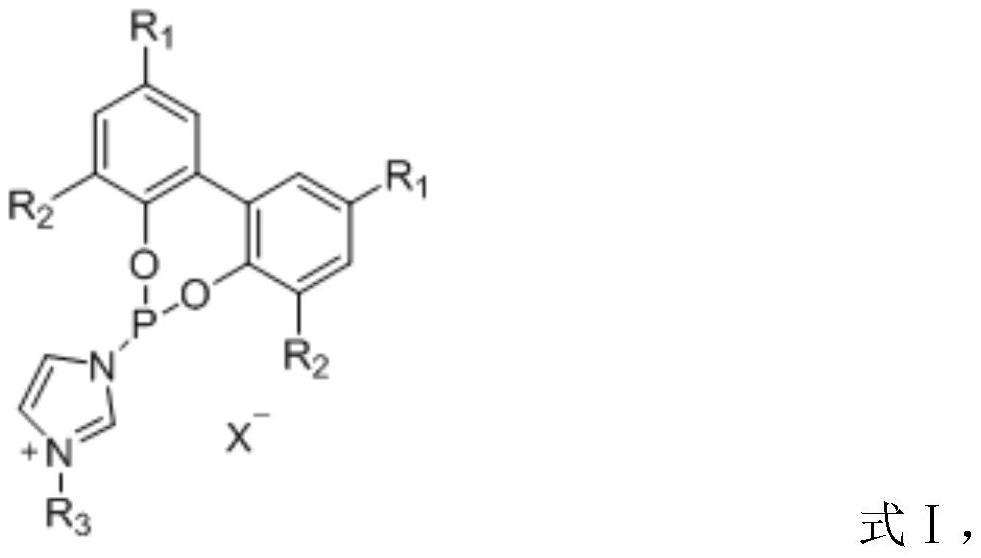
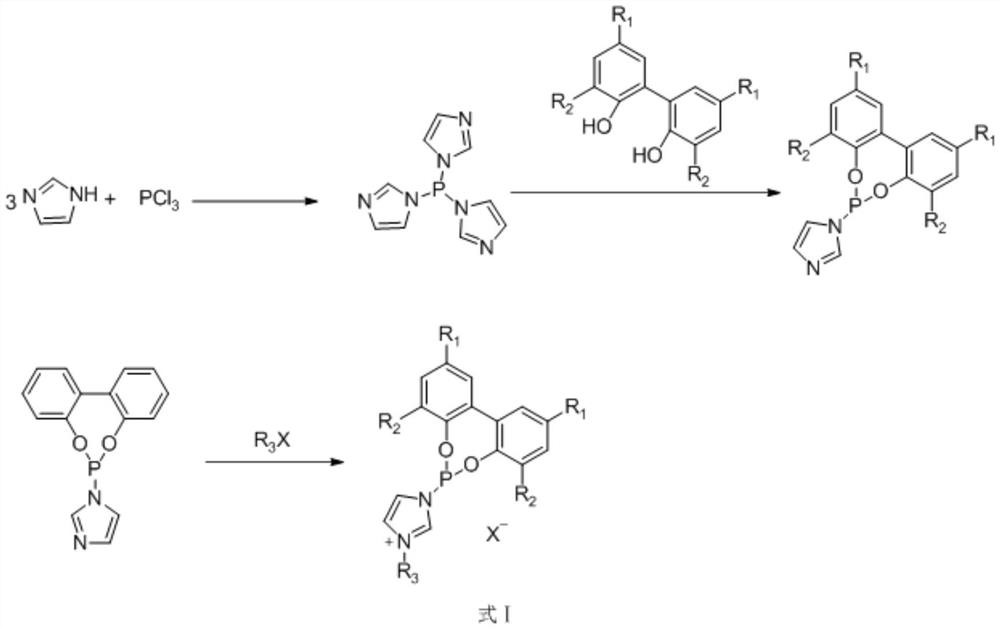
Preparation method and application of ionic phosphoramidite ligand
The technology of phosphonamide and monodentate phosphonamide is applied in the field of preparation of ionic phosphonamide ligands, which can solve the problem of limited research on phosphonamide ligands, and achieves efficient preparation, functional modification, good Application prospect, favorable effect of reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation method of the ionic monodentate phosphoramidite with structural formula A and its application in the hydroformylation reaction of butene:
[0038]
[0039] Dissolve 2.04 g of imidazole in 50 mL of ultra-dry dichloromethane under the protection of nitrogen, add 0.43 mL of phosphorus trichloride dropwise under ice bath, react for 30 minutes and filter, and protect the filtrate with nitrogen. Dissolve 0.93 g of biphenol in 10 mL of ultra-dry dichloromethane, add the solution dropwise to the above filtrate, react at room temperature for 1 hour, filter, and spin to dry the solvent. The obtained product is purified by column chromatography, and the eluent is ethyl acetate : n-hexane = 1:10, to obtain monodentate phosphoramidite product. Under the protection of nitrogen, 1.41 g of the obtained monodentate phosphoramidite was dissolved in 20 mL of toluene, 0.52 mL of n-chlorobutane was added, the temperature was raised to 110 ° C and refluxed for 12 h, after ...
Embodiment 2
[0042] The preparation method of the ionic monodentate phosphoramidite whose structural formula is B and its application in the hydroformylation reaction of butene:
[0043]
[0044] Dissolve 2.04 g of imidazole in 50 mL of ultra-dry dichloromethane under the protection of nitrogen, add 0.43 mL of phosphorus trichloride dropwise under ice bath, react for 30 minutes and filter, and protect the filtrate with nitrogen. Dissolve 0.93 g of biphenol in 10 mL of ultra-dry dichloromethane, add the solution dropwise to the above filtrate, react at room temperature for 1 hour, filter, and spin to dry the solvent. The obtained product is purified by column chromatography, and the eluent is ethyl acetate : n-hexane = 1:10, to obtain monodentate phosphoramidite product. Under the protection of nitrogen, 1.41 g of the obtained monodentate phosphoramidite was dissolved in 20 mL of toluene, 0.61 g of 1,3-propane sultone was added, the temperature was raised to 110°C and refluxed for 12 hou...
Embodiment 3
[0047] The preparation method of ionic monodentate phosphoramidite with structural formula C and its application in butene hydroformylation reaction:
[0048]
[0049] Dissolve 2.04 g of imidazole in 50 mL of ultra-dry dichloromethane under the protection of nitrogen, add 0.43 mL of phosphorus trichloride dropwise under ice bath, react for 30 minutes and filter, and protect the filtrate with nitrogen. Dissolve 2.05 g of 3,3'-5,5'-tetra-tert-butyl-2,2'-biphenol in 10 mL of ultra-dry dichloromethane, add the solution dropwise to the above filtrate, react at room temperature for 1 hour, and filter , and the solvent was spin-dried, and the obtained product was purified by column chromatography, and the eluent was ethyl acetate:n-hexane=1:10, to obtain a monodentate phosphonite amide product. Under the protection of nitrogen, 2.53 g of the obtained monodentate phosphoramidite was dissolved in 20 mL of toluene, 0.61 g of 1,3-propane sultone was added, the temperature was raised t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com