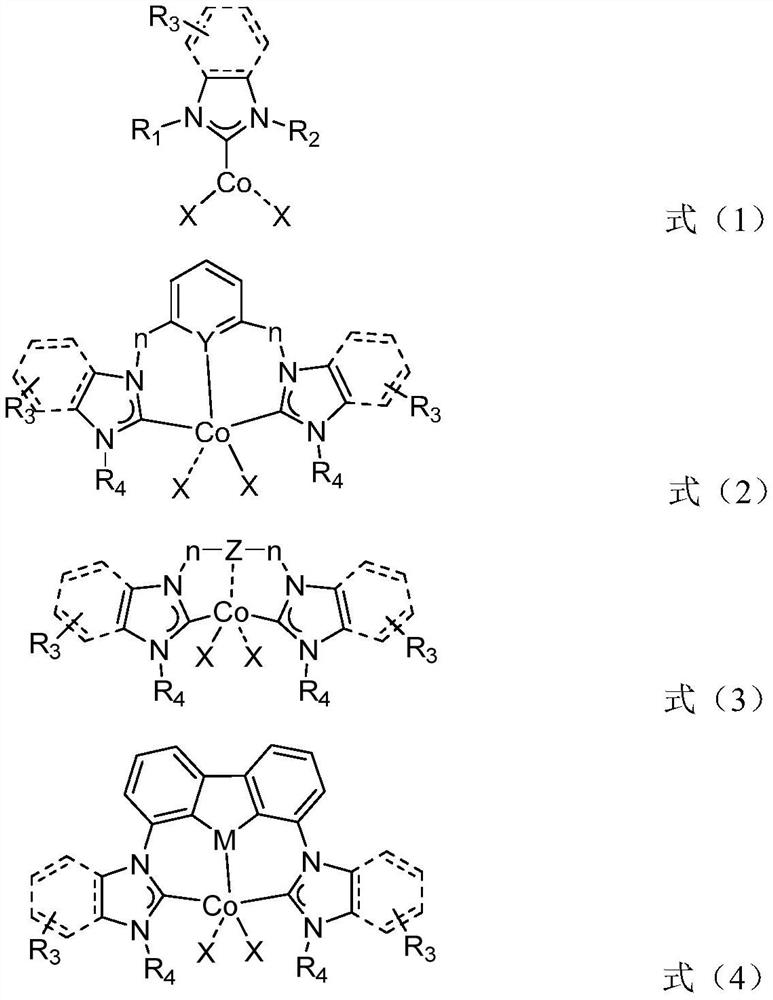
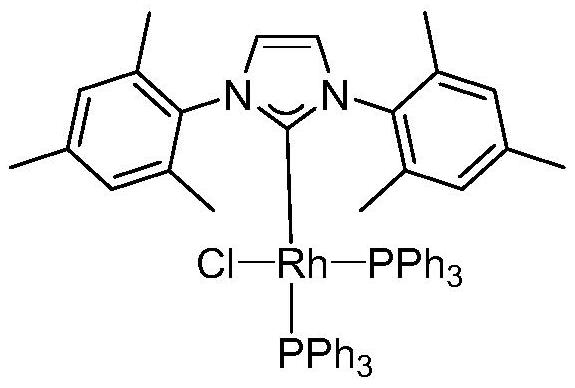
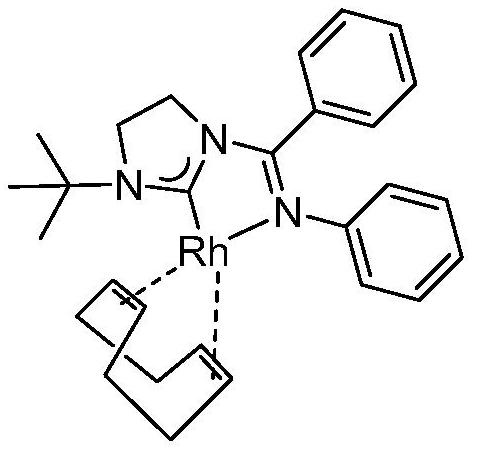
Method for synthesizing valeraldehyde
A technology of valeraldehyde and mixed gas, applied in chemical instruments and methods, preparation of organic compounds, preparation of carbon-based compounds, etc., can solve the problems of limited industrial application process, harsh reaction conditions, high price, etc., and achieve high industrial application prospects , The synthesis process is simple and the conditions are mild
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Add 0.04mmol of cobalt complex (19.4mg) catalyst 1 into a 100mL reactor, add 40mL of toluene solvent and use CO / H 2 Mixed gas (molar ratio 1:1) was used to replace the reactor. After three replacements, 20 mmol (1.72 g) of n-butene was added. After adding n-butene, CO / H with a molar ratio of 1:1 was charged. 2 Mix gas to 2MPa, and react at 110°C. After 24 hours, the reaction was completed, the reaction kettle was cooled, and the residual gas was released carefully under ventilated conditions. An internal standard was added to the solution after the reaction, and gas chromatography analysis was carried out. The yield of n-valeraldehyde obtained was 21%, and n-butene The conversion rate was 28%, and the positive-to-differential ratio was 3:1.
Embodiment 2
[0036]
[0037] Add 0.025mmol of cobalt complex (16.98mg) catalyst 2 into a 100mL reactor, add 40mL of toluene solvent and use CO / H 2 Mixed gas (molar ratio 1:1) was used to replace the reactor. After three replacements, 20mmol (1.72g) of n-butene was added. After adding n-butene, CO / H with a molar ratio of 1:1 was charged. 2Mix gas to 3MPa, and react at 120°C. After 24 hours, the reaction was completed, the reaction kettle was cooled, and the residual gas was released carefully under ventilated conditions. An internal standard was added to the solution after the reaction, and gas chromatography analysis was carried out. The yield of n-valeraldehyde obtained was 36%, and n-butene The conversion rate was 45%, and the positive-to-isotropic ratio was 3.8:1.
Embodiment 3
[0039]
[0040] Add 0.02mmol of cobalt complex (15.92mg) catalyst 3 into a 100mL reaction kettle, add 40mL of toluene solvent and use CO / H 2 Mixed gas (molar ratio 1:1) was used to replace the reactor. After three replacements, 20 mmol (1.72 g) of n-butene was added. After adding n-butene, CO / H with a molar ratio of 1:1 was charged. 2 Mixed gas to 3MPa, react at 140°C. After 36 hours, the reaction was finished, and the reaction kettle was cooled, and the remaining gas was released carefully under ventilated conditions. An internal standard was added to the solution after the reaction, and gas chromatography analysis was carried out. The yield of n-valeraldehyde obtained was 61%, and n-butene The conversion rate is 70%, and the positive-to-isotropic ratio is 6:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com