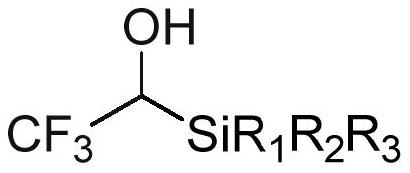
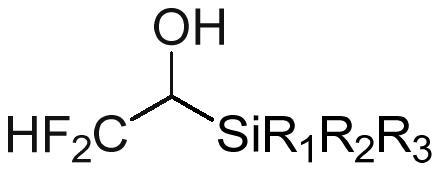
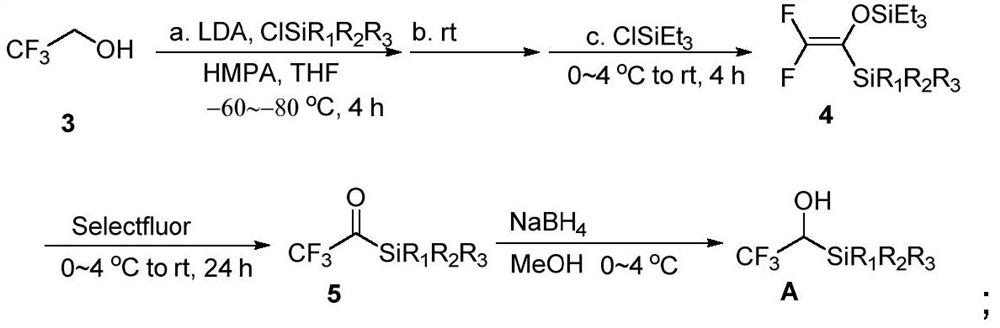
Trifluoroethanol/difluoroethanol reagent as well as preparation method and application thereof
A technology of trifluoroethanol and difluoroethanol, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of hydroxyl compounds, etc., can solve the problems of difficult to control regioselectivity and unstable aldehyde compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Ethyl 5,5,5-trifluoro-4-hydroxy-2-methylenevalerate, the synthetic route and preparation method are as follows:
[0071]
[0072] Into a dry 10 mL Schlenk tube under a nitrogen atmosphere, add Mn(OAc) 2 4H 2 O (14.7mg, 0.06mmol, 20mol%), 7a (152.4mg, 0.6mmol, 2.0 equiv), DCM (3mL, 0.1 M), 1a (70.2mg, 0.3mmol)) and TBPB (145.7mg, 0.75mmol, 2.5 equivalents), then sealed the tube, heated to 70°C and stirred for 18h, then added TBAF in an ice-water bath at 5°C, stirred for 0.5h, quenched the reaction mixture with water (2mL), and extracted with DCM (3×10mL) , the organic phases were combined and washed with brine, washed with Na 2 SO 4 Dry, hang dry using a rotary evaporator. The crude product was purified by silica gel column chromatography (200×300 mesh), and eluted with PE / EA (20 / 1~10 / 1, v / v) (PE: petroleum ether, EA: ethyl acetate) to obtain 53 mg (product Yield 71%) the target compound as a yellow oil. The product is tested, and the test results are as follows...
Embodiment 2
[0088] 5,5,5-Trifluoro-4-hydroxy-2-methylene-1-phenylpentan-1-one, the synthetic route and preparation method are as follows:
[0089]
[0090] Into a dry 10 mL Schlenk tube under a nitrogen atmosphere, add Mn(OAc) 3 2H 2 O (16.1mg, 0.06mmol, 20mol%), 7b (257.4mg, 0.9mmol, 3.0eq), DCM (3mL, 0.1M), 1a (70.2mg, 0.3mmol)) and TBPB (145.7mg, 0.75mmol, 2.5 equivalents), then seal the sealed tube, heat to 70°C and stir for 14h, after that, in 5°C ice-water bath, add TBAF, stir for 0.5 hour, quench the reaction mixture with water (2mL), and use DCM (3×10mL) extracted, the organic phases were combined and washed with brine, washed with Na 2 SO 4 Drying, using a rotary evaporator to hang dry, the crude product was purified by silica gel column chromatography (200 × 300 mesh), and eluted with PE / EA (20 / 1~10 / 1, v / v) to obtain 53mg (yield 71% ) target compound as a yellow oil. The product is tested, and the test results are as follows:
[0091] R f =0.40 (PE / EA=5 / 1, v / v).
[00...
Embodiment 3
[0098] 1-([[1,1'-biphenyl]-4-yl)-5,5,5-trifluoro-4-hydroxy-2-methylenepentyl-1-one
[0099]
[0100] Into a dry 10 mL Schlenk tube under a nitrogen atmosphere, add Mn(OAc) 3 2H 2 O (16.1 mg, 0.06 mmol, 20 mol%), 7c (326.2 mg, 0.9 mmol, 3.0 equiv), DCM (3 mL, 0.1 M), 1a (70.2 mg, 0.3 mmol)) and TBPB (145.7 mg, 0.75 mmol, 2.5 eq), then seal the sealed tube, heat to 70°C and stir for 14h, after that, add TBAF in 5°C ice-water bath, stir for 0.5h, quench the reaction mixture with water (2mL), and extract with DCM (3×10mL) , the organic phases were combined and washed with brine, washed with Na 2 SO 4 Dry, hang to dry using a rotary evaporator, the crude product is purified by silica gel column chromatography (200×300 mesh), and eluted with PE / EA (20 / 1~10 / 1, v / v) to obtain 74.9 mg (yield 78 %) target compound as a white solid. The product is tested, and the test results are as follows:
[0101] R f =0.50 (PE / EA=5 / 1, v / v), boiling point (mp): 69-71°C.
[0102] NMR spectru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com