Salmonella paratyphi A polysaccharide modified nanoparticles and application thereof
A salmonella and paratyphoid technology, applied in the field of fusion proteins, can solve the problems of high cost, non-uniform products, reaching nano-level, etc., and achieve the effect of improving the response ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
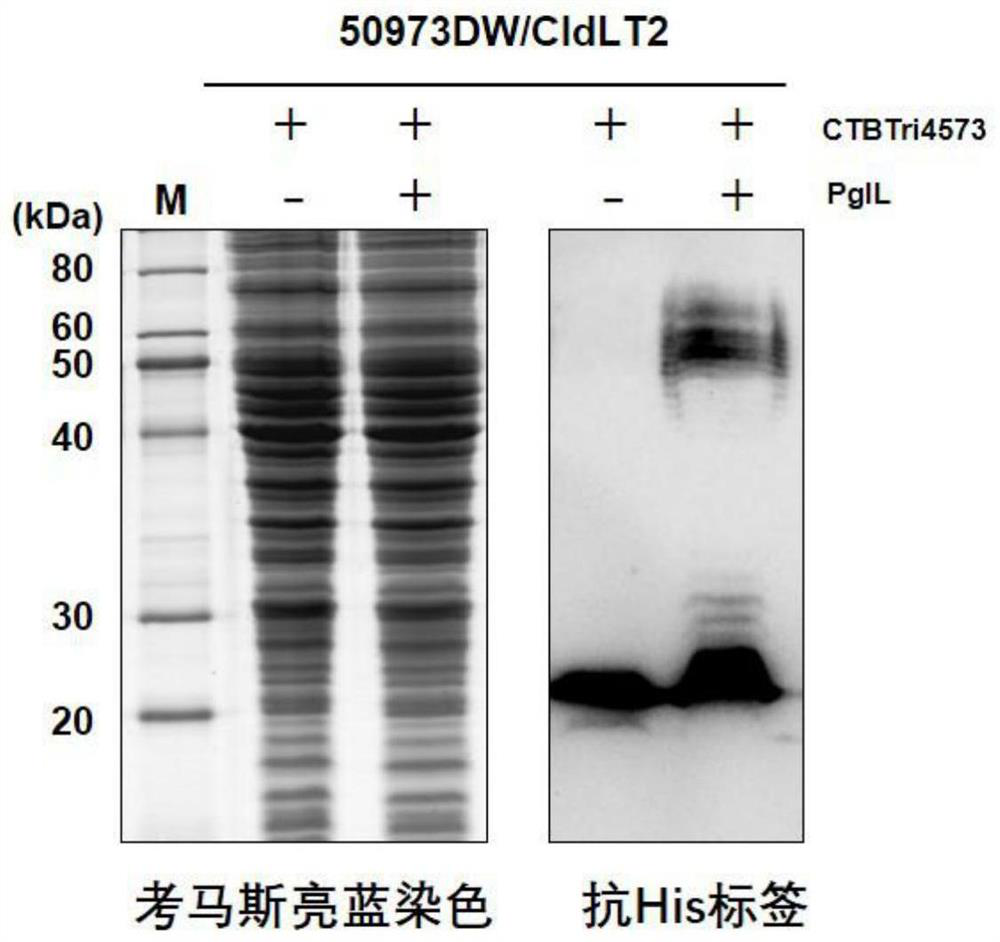
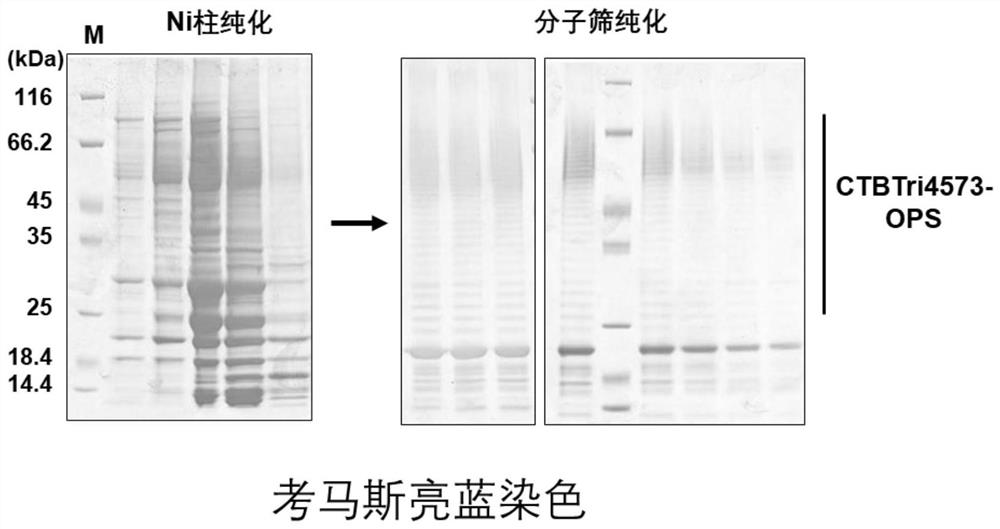
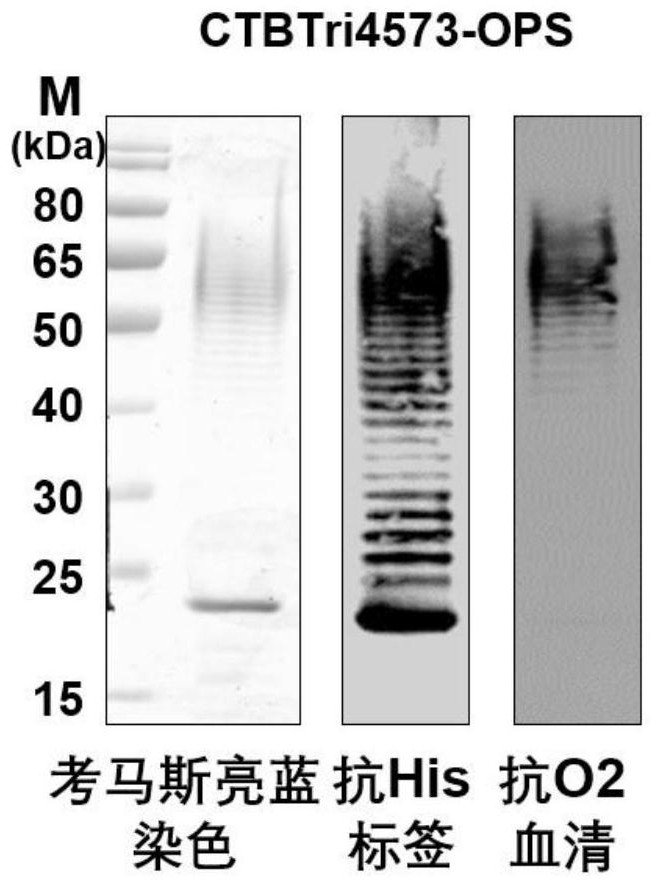
[0057] Example 1. Preparation of Nanoscale Bacterial Polysaccharide Conjugated Vaccines by Biological Method
[0058] 1) Construction of the expression vector of the glycosylated fusion protein CTBTri4573
[0059] Construction of Neisseria meningitidis glycosyltransferase PglL expression vector: The amino acid sequence of Neisseria meningitidis glycosyltransferase PglL (GeneBank: JN200826.1) is shown in SEQ ID No.1, and its coding sequence is shown in SEQ ID No. 180-1994 nucleotides of .2. The 1st-6th nucleotide of SEQ ID No.2 is the XbaI recognition site, and the 105th-2240th nucleotide of SEQ IDNo.2 is the sequence of the PglL expression cassette. In the PglL expression cassette, the expression of PglL is controlled by tac The promoter is activated, and the expression cassette is named tacpglL. Wherein, the 105th-133rd nucleotide of SEQ ID No.2 is the sequence of the tac promoter, the 180th-1994th nucleotide is the coding sequence of Neisseria meningitidis glycosyltransfer...
Embodiment 2
[0081] Example 2. Evaluation of animal immunity
[0082] 1) Serum titer after immunization of mice
[0083] Forty 6-week-old female Balb / c mice were randomly divided into 4 groups: PBS group (10 mice), OPS group (10 mice), CTB4573-OPS group (10 mice) and CTBTri4573-OPS group (10 mice). Only). Female Balb / c mice in OPS group, CTB4573-OPS group and CTBTri4573-OPS group were injected with OPS (Design and production of conjugate vaccines against S. ParatyphiAusing an O-linked glycosylation system invivo. NPJ Vaccines. 2018, 3: 4), Glycosylation obtained in CTB4573-OPS (Design and production of conjugate vaccines against S. ParatyphiAusing an O-linked glycosylation system in vivo. NPJ Vaccines. 2018, 3:4) and CTBTri4573-OPS (corresponding to step 5 in Example 1) mice in each group were subcutaneously injected with 2.5 μg of polysaccharide as measured by the polysaccharide content; they were immunized on the 1st, 15th, and 29th day, respectively, and the tail blood was collected 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com