Method for fusion expression of antibacterial peptide by using SUMO
A technology of antimicrobial peptides and fusion proteins, applied in cationic antimicrobial peptides, hybrid peptides, fusion with degradation motifs, etc., can solve problems such as degradation, small molecular weight of antimicrobial peptides, and toxicity of host bacteria, so as to reduce toxicity and improve folding Effect of rate and expression yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Design and Screening of Antimicrobial Peptides
[0026] The invention uses the antibacterial peptide LL-37 as the starting polypeptide, and designs a new antibacterial peptide LLv by adding hydrophobic residues and positively charged amino acids. Compared with LL-37, LLv has increased stability and elongated α-helical structure. The amino acid sequence of the modified antimicrobial peptide is as follows (SEQ ID NO: 1): LLGDFFKKSKEK IGKEFKRIVQRIKDFLRNLVWKTEK.
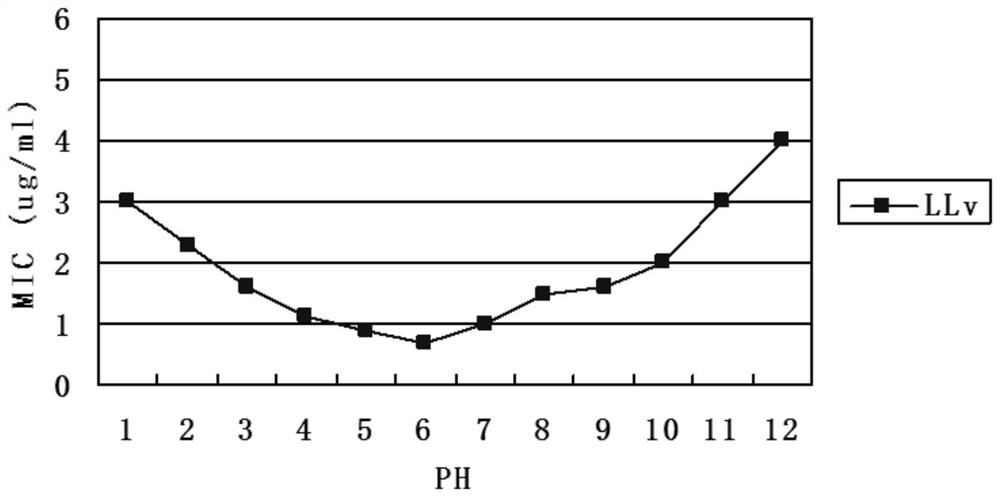
[0027] By adding hydrophobic residues and positively charged amino acids, the new antimicrobial peptide LLv has increased thermostability compared with LL-37. Antimicrobial peptide LLv has certain activity at pH 2.0-12.0. The antibacterial activity did not change significantly after boiling water bath for 40 minutes, and the structure and antibacterial activity of antimicrobial peptide LLv could not be destroyed under high pressure at 121°C for 21 minutes.
Embodiment 2
[0028] Embodiment 2: Physicochemical property analysis antibacterial test of antimicrobial peptide LLv
[0029] 1. Use the dilution method antibacterial test to detect the antibacterial performance of the antimicrobial peptide LLv. The specific steps are as follows:
[0030] 1) Preparation of bacterial solution, inoculate the experimental bacteria (Escherichia coli ATCC25922, avian Escherichia coli O1, O2, Pasteurella, Bacillus subtilis, Staphylococcus aureus ATCC25923) into MH broth, place in a shaker incubator, and incubate at 37°C 12-18h, so that the bacteria are in the logarithmic growth phase. Then dilute the bacterial solution with sterile saline to the desired number of bacteria.
[0031] 2) Determination by microdilution method
[0032] Take a sterile 96 polystyrene microwell plate test tube, add the antimicrobial peptide solution sterilized by filtration to the MH broth medium in sequence, so that the final concentrations of the first hole to the tenth hole are 276 ...
Embodiment 3
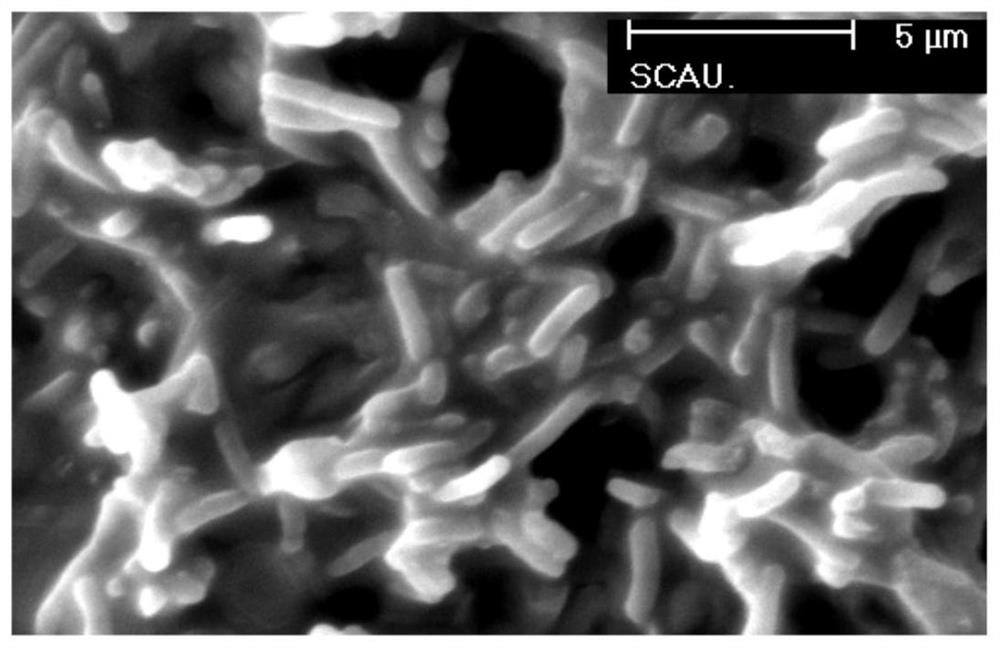
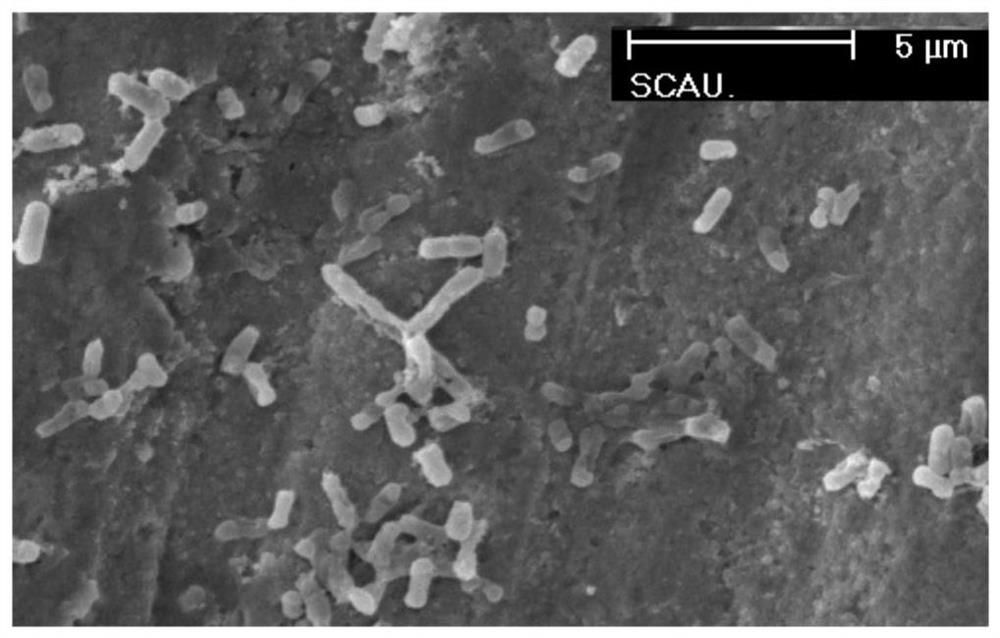
[0041] Embodiment 3: the scanning electron microscope examination of the antimicrobial peptide LLv processed bacterium
[0042]Incubate the antimicrobial peptide LLv at a concentration of 2 μg / ml with Escherichia coli ATCC25922 at 37°C for 60 minutes, then collect the bacterial solution, centrifuge at 3000 r / min for 10 minutes, three times, discard the supernatant, and replace with 0.06 mol / L phosphate buffer (PH7.2) rinse 3 times, retain the precipitate, add 2.5% glutaraldehyde to make bacterial suspension, fix at 4°C for 4h, centrifuge at 3000r / min, 10min each time, three times. The detailed method of preparing samples of normal bacteria control group is the same as that of antimicrobial peptide group. Finally, PBS was used to suspend the bacteria to make a suspension. The bacteria were then allowed to sink freely, dried, and sputtered with gold under vacuum. Finally, the bacteria structure was observed by scanning electron microscope, and the working voltage was 10KV.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com