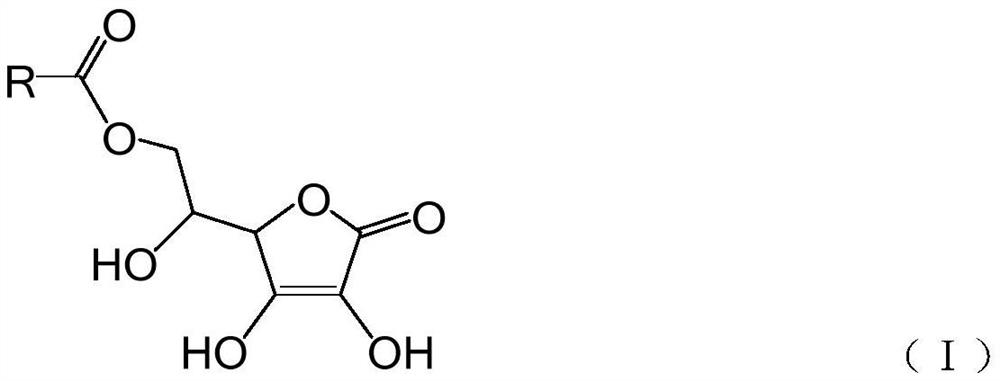
Green synthesis method of vitamin C higher fatty acid ester
A technology for higher fatty acids and fatty acid esters, applied in the directions of organic chemistry, chemical recycling, etc., can solve the fundamental problems of inability to solve a large amount of waste acid water, unfavorable to large-scale production, and high production costs, achieving low cost, saving production costs, The effect of less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation of embodiment 1 vitamin C palmitate
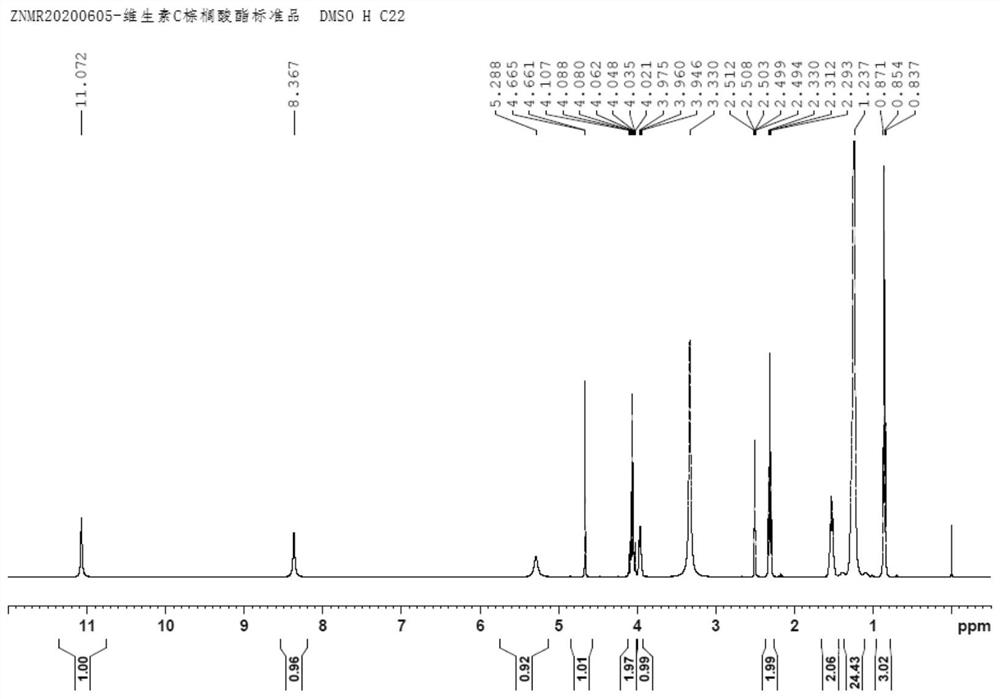
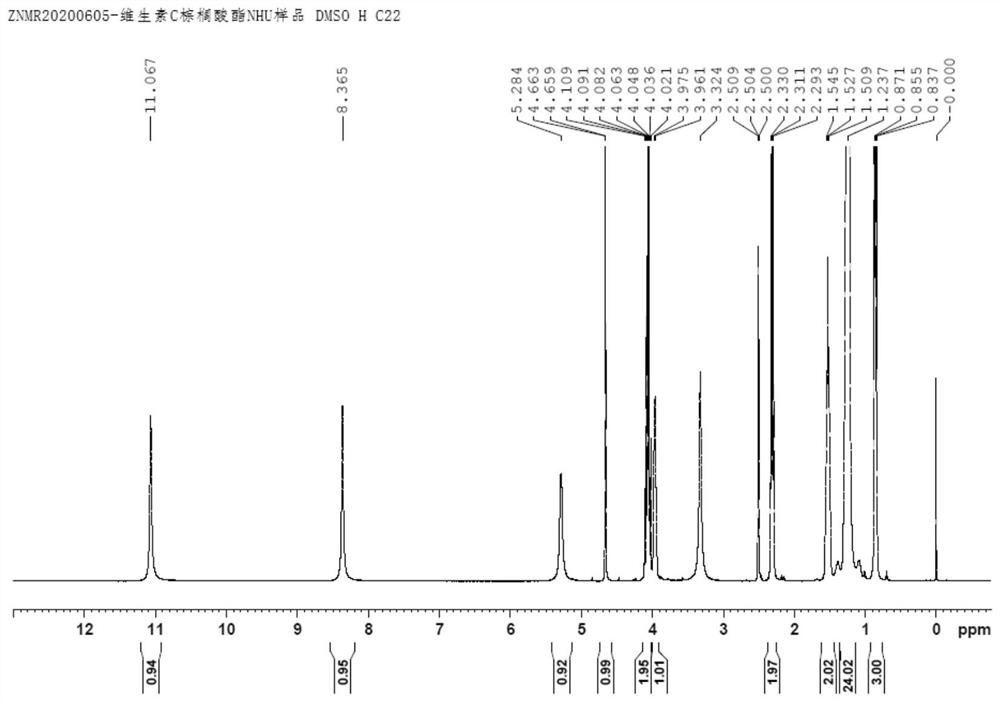
[0050] Put palmitic anhydride (50g, 0.101mol), vitamin C (17.61g, 0.100mol) and DMAP (0.02g) into a 250mL four-neck flask with a reflux and heating device, slowly raise the temperature to 70°C, react for 8 hours, and take a sample After HPLC detection, vitamin C residue ≤ 0.5% is qualified, stop heating, add n-hexane (200g) dropwise, drop it in 0.5 hour, cool down to 5-10°C, stir for 1 hour, filter, and cool and normalize with 0-5°C. The filter cake was washed 3 times with hexane and dried to obtain the product vitamin C palmitate (41.12 g, 0.0992 mol), which was white crystals in appearance, HPLC purity: 99.30%, and molar yield: 98.50%. H NMR spectrum see figure 2 , which is consistent with the standard product; in addition, the national standard GB1886.230-2016 of vitamin C palmitate is used for the HPLC detection method, the peak time is consistent, and the product structure can be confirmed.
Embodiment 2
[0051] The preparation of embodiment 2 vitamin C palmitate (different reaction temperature)
[0052] Put palmitic anhydride (50g, 0.101mol), vitamin C (17.61g, 0.100mol) and DMAP (0.02g) into a 250mL four-neck flask with a reflux and heating device, slowly raise the temperature to 85°C, react for 8 hours, and take a sample After HPLC detection, vitamin C residue ≤ 0.5% is qualified, stop heating, add n-hexane (200g) dropwise, drop it in 0.5 hour, cool down to 5-10°C, stir for 1 hour, filter, and cool and normalize with 0-5°C. The filter cake was washed 3 times with hexane and dried to obtain the product vitamin C palmitate (41.16 g, 0.0993 mol), which was white crystals in appearance, HPLC purity: 99.24%, and molar yield: 98.54%.
Embodiment 3
[0053] The preparation of embodiment 3 vitamin C palmitic acid esters (raw materials in different ratios)
[0054] Put palmitic anhydride (55g, 0.111mol), vitamin C (17.61g, 0.100mol) and DMAP (0.02g) into a 250mL four-neck flask with a reflux and heating device, slowly raise the temperature to 70°C, react for 8 hours, and take a sample After HPLC detection, vitamin C residue ≤ 0.5% is qualified, stop heating, add n-hexane (200g) dropwise, drop it in 0.5 hour, cool down to 5-10°C, stir for 1 hour, filter, and cool and normalize with 0-5°C. The filter cake was washed 3 times with hexane and dried to obtain the product vitamin C palmitate (41.31 g, 0.0993 mol), which was white crystals in appearance, HPLC purity: 99.67%, and molar yield: 99.33%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com