New pharmaceutical application of BML-28-4
A BML-284, 1. BML-284 technology, applied in the field of medicine, can solve the problem of no BML-284 endogenous myocardial cell proliferation, etc., and achieve the effect of promoting myocardial cell proliferation and promoting proliferation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
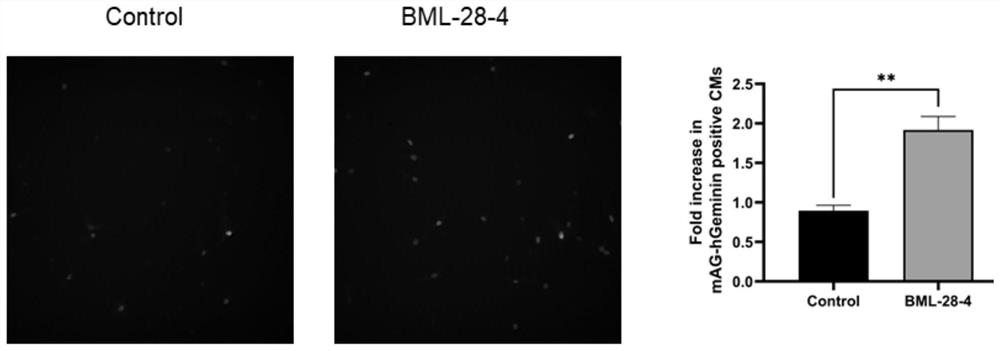
[0040] Embodiment 1, the in vitro test of BML-284 promoting the proliferation of rat cardiomyocytes
[0041] (1) Culture of Cardiomyocytes of SD Rats
[0042] Cardiomyocytes from SD rats born 3 days old were isolated and cultured in DMEM high glucose medium (Hyclone) + 5% horse serum (GIBCO) at 37 degrees in a 5% carbon dioxide incubator.
[0043] (2) Experimental grouping and processing
[0044] Isolate cardiomyocytes from SD rats, add 5% horse serum (GIBCO) + DMEM high-glucose medium (Hyclone), and add cytarabine (final concentration 20umol / L) to inhibit the growth of non-cardiomyocytes. Infect cTnT-mAG-hGeminin (1 / 110) virus (MOI value of virus infection=100) after 48 hours of adherence, after another 24 hours, replace with DMEM culture medium containing 0.5% FBS, add drugs in groups, and group as follows:
[0045] a. Experimental group: treated with BML-284 (the final concentration in the medium is 2 μmol / L) for 24 hours.
[0046] b. Blank control group: add the same am...
Embodiment 2
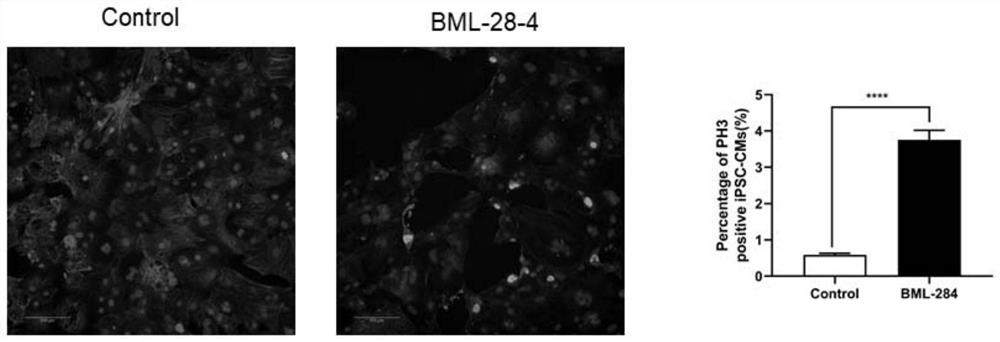
[0051] Example 2. In vitro test of BML-284 promoting the proliferation of human iPSC-derived cardiomyocytes
[0052] Human induced pluripotent stem cell-induced cardiomyocytes (hiPSC-derived cardiomyocytes) were treated with BML-284 for 48 hours, RPMI1640+DMSO was used as the control (control), washed three times with PBS after 48 hours, 4% PFA (paraformaldehyde) at room temperature Fix for 15 min, wash with PBS three times, block with 1% BSA / PBS / 0.1% Triton for 1 h at room temperature, add primary antibody after 1 h: Anti-Cardiac Troponin T (1:300, abcam, mouse, ab8295), Phospho-Histone H3 (Ser10) Antibody (1:300, CST, rabbit, 9701S), overnight at 4°C, wash 4 times with 1% BSA / PBS / 0.02% Tween, add secondary antibody: goat anti-rabbit IgG (H+L) highly cross-adsorbed secondary Antibody (1:500, Invitrogen, A32731), goat anti-mouse IgG (H+L) highly cross-adsorbed secondary antibody (1:500, Life technologies, A21424), placed at room temperature for 1 h, washed with PBS / 0.02% Tween...
Embodiment 3
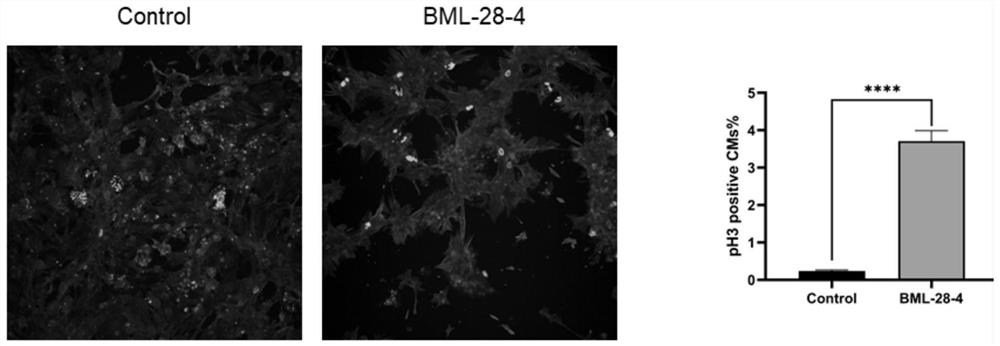
[0054] Example 3. In vitro test of BML-284 promoting neonatal rat cardiomyocytes to enter the cell cycle
[0055] Isolate rat cardiomyocytes born 3 days old, add BML-284 (the final concentration in the culture medium is 2 μ mol / l), use DMEM+DMSO as the control (control), wash three times with PBS after 48 h, 4% PFA (polymer Formaldehyde) fixed at room temperature for 15 min, washed three times with PBS, blocked at room temperature with 1% BSA / PBS / 0.1% Triton for 1 h, and added primary antibody after 1 h: Anti-α-Actinin (1:300, sigma, mouse, A7811), Phospho-Histone H3 (Ser10) antibody (1:300, CST, rabbit, 9701S), Anti-Ki67 (1:300, abcam, rabbit, ab15580) overnight at 4°C, wash 4 times with 1% BSA / PBS / 0.02% Tween, add two Anti: goat anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibody (1:500, Invitrogen, A32731), goat anti-mouse IgG (H+L) highly cross-adsorbed secondary antibody (1:500, Life technologies, A21424), placed at room temperature for 1 hour, washed 3 times ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com