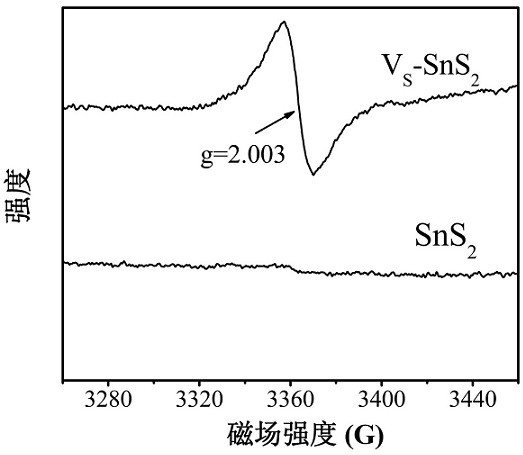
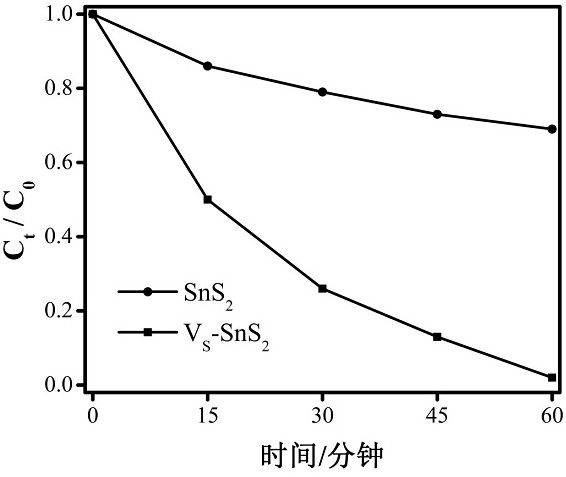
Preparation of SnS2 nanosheet with S vacancy and application of SnS2 nanosheet in photodegradation of Cr (VI)
A nanosheet and photodegradation technology, which is applied in the fields of environmental protection and pollutant degradation, can solve the problems of low reduction efficiency and slow reduction speed, and achieve the effects of low cost, improved removal rate, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) SnS without S vacancies 2 The preparation method of nanosheet: carry out successively according to the following steps:
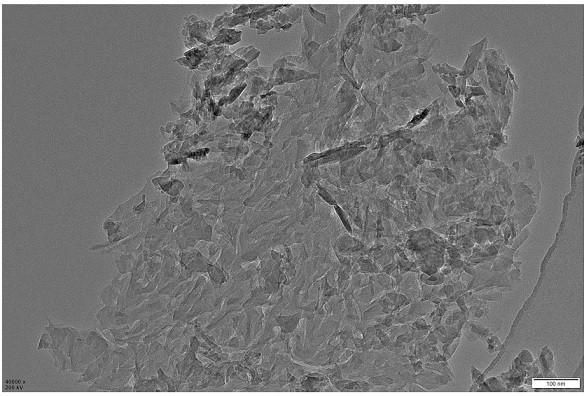
[0039] SnCl with a concentration of 2g / L 4 ·5H 2 O and thioacetamide with a concentration of 1.7 g / L were added to 40 mL of ethylene glycol, and ultrasonicated for 0.5 h at room temperature, and the thioacetamide solution was added to the SnCl 4 ·5H 2 O solution was stirred for 1 h, and the resulting suspension was transferred to a 100 mL high-pressure hydrothermal reactor with a polytetrafluoroethylene liner, and heated at 140 °C for 10 h; after cooling to room temperature, a yellow suspension was collected, and the yellow suspension was used for Alternately washed with ethanol and deionized water for several times, and finally dried in a vacuum oven at 60°C for 8 hours to obtain SnS 2 nanosheets; attached figure 1 This is a good illustration of its ultra-thin structural features.
[0040] (2) SnS without S vacancies 2 The method for phot...
Embodiment 2
[0045] (1) Preparation method of SnS2 nanosheets without S vacancies: follow the steps below:
[0046] SnCl with a concentration of 3g / L 4 ·5H 2 O and thioacetamide with a concentration of 3.4 g / L were added to 40 mL of ethylene glycol, sonicated at room temperature for 1 h, and the thioacetamide solution was added to the SnCl 4 ·5H 2 O solution was stirred for 1.5h, and the resulting suspension was transferred to a 100mL high-pressure hydrothermal reactor lined with Teflon, and heated at 160°C for 12h; after cooling to room temperature, a yellow suspension was collected, yellow suspension Alternately wash with ethanol and deionized water several times, and finally dry in a vacuum oven at 60°C for 8 hours to obtain SnS 2 Nanosheets.
[0047] (2) Use the method described in Example 1 to photodegrade the hexavalent chromium Cr(VI) of the sample obtained in the example. The test absorbance is 0.422, and the degradation rate is calculated to be 27%.
Embodiment 3
[0049] (1) SnS without S vacancies 2 The preparation method of nanosheet: carry out successively according to the following steps:
[0050] SnCl with a concentration of 4g / L 4 ·5H 2 O and thioacetamide with a concentration of 10.2 g / L were added to 40 mL of ethylene glycol, and ultrasonicated for 2 h at room temperature, and the thioacetamide solution was added to the SnCl 4 ·5H 2 O solution was stirred for 2h, and the resulting suspension was transferred to a 100mL high-pressure hydrothermal reactor lined with polytetrafluoroethylene, and heated at 180°C for 16h; after cooling to room temperature, a yellow suspension was collected, and the yellow suspension was used for Alternately washed with ethanol and deionized water for several times, and finally dried in a vacuum oven at 60°C for 8 hours to obtain SnS 2 Nanosheets.
[0051] (2) Use the method described in Example 1 to photodegrade the hexavalent chromium Cr(VI) of the sample obtained in the example. The test absorb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com