Crystalline forms of a lta4h inhibitor
A technology of crystal form and amino group, which is applied in the field of crystal form of LTA4H inhibitors, can solve the problem of toxicity of polymorphs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0203] abbreviation:
[0204] IT: internal temperature
[0205] Tj: reactor jacket temperature
[0206] Tr: reaction temperature
[0207] THF: Tetrahydrofuran
[0208] Rpm: revolutions per minute
example 1
[0209] Example 1: Form H B preparation of
[0210]
[0211] Into a 2500 L glass-lined reactor was charged Int-1 (55 kg) and toluene (810 kg) and the mixture was stirred (55 rpm, anchored). 31% HCl (232 kg) was added. The discharge pressure of the reactor was set at 2000 mbar. The internal temperature was adjusted to 65 °C and stirred (60 rpm) for 11 h 29 min at an internal temperature (IT) between 65 °C and 66 °C. The internal temperature was then adjusted to 35°C. Then through the Dean-Stark separator-like device (IT max =44° C.) 82 kg of aqueous solution were separated under reduced pressure and the internal temperature was adjusted to about 20°C. The system was stirred at 19°C to 24°C for 80 min. The solid was collected by centrifugation. The wet cake was washed with toluene (50 kg, 20 mL of antistatic agent added) and dichloromethane (50 kg). The solid was dried at 195 mbar for 8 h 26 min with a solution of sodium bisulfite from the company JT Baker set at 60°...
example 2
[0217] Example 2: Preparation of Hydrate Form B
[0218]
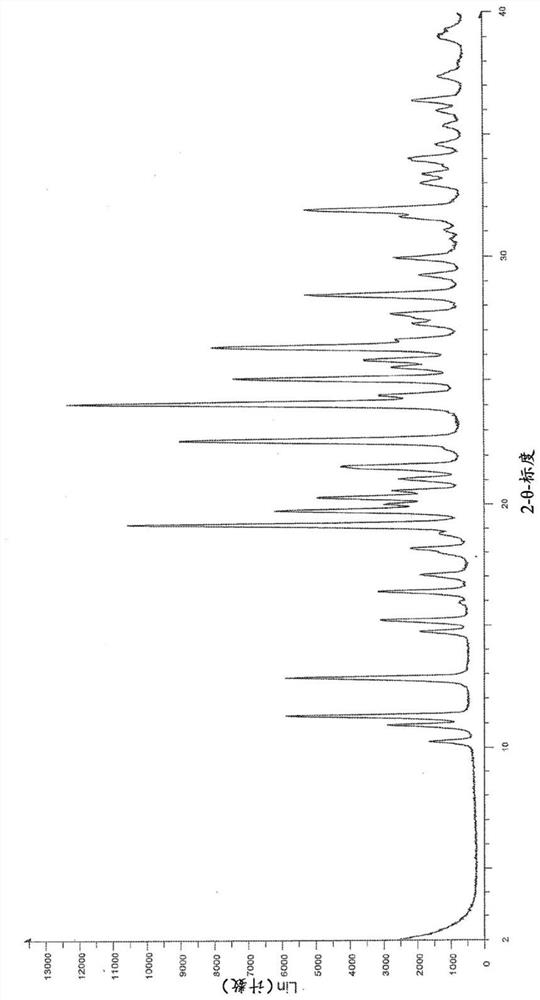
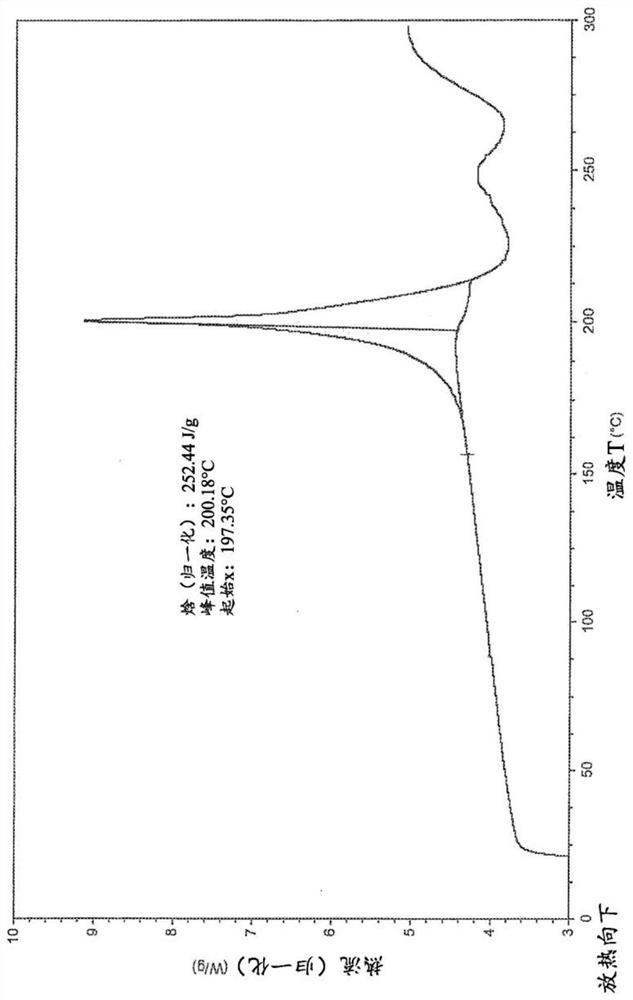
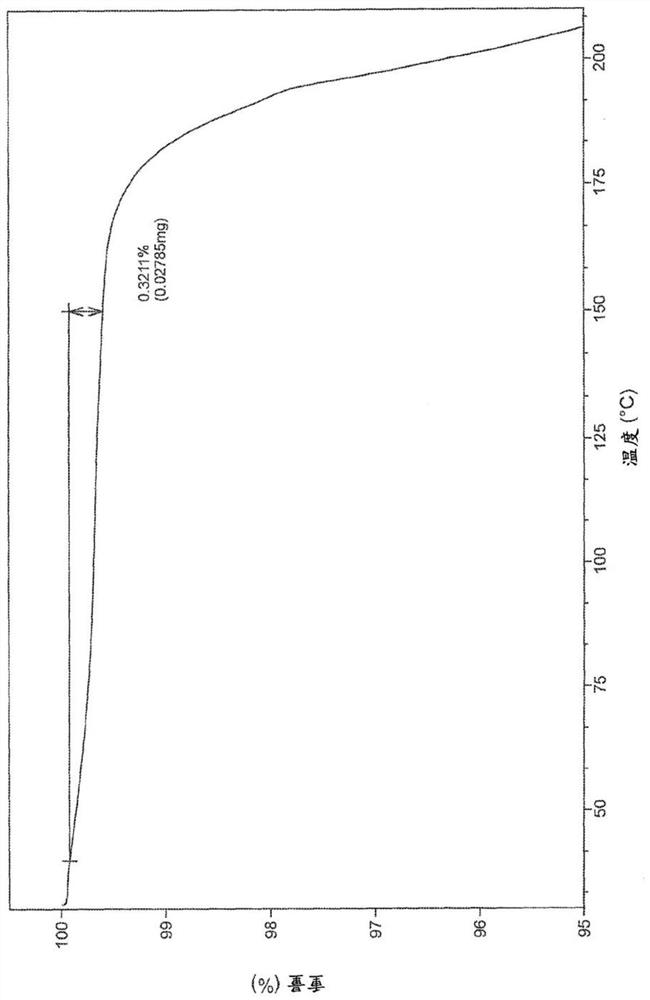
[0219] The first method: 505mg H B Weigh into a 20ml glass vial and add 6mL of methanol. The slurry was heated to 50 °C and stirred using a magnetic stirrer for 4 days. The suspension was cooled to room temperature and filtered. The recovered solid was dried under vacuum at 40 °C for 2.5 h. The white solid was analyzed by XRPD, DSC and TGA (respectively Figure 1-3 ).
[0220] The second method: stirring H in water / MeOH (2:8, 1:9, 1:2 v / v) at a temperature above 60 °C B Type B will also lead to Type B. For example, 20.7g H B type (dry weight) was added to the solution containing 810mL MeOH and 90mL H 2 O in a premixed solvent mixture. The resulting mixture was heated to 68 °C for 36 h, then cooled to 25 °C. The mixture was stirred for another 2h and filtered. The filter cake was dried under vacuum to give Form B (15.8 g, 76% yield) as a white powder.
[0221] M / z=393.1[M+H] + , Rt=2.47min (UPLC-MS con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com